第 1讲 概述
Heads Up!
The immune system is a "team effort," involving many different players. These players can be divided roughly into two groups: those that are members of the innate immune system team and those that are part of the adaptive immune system. Importantly, these two groups work together to provide a powerful defense against invaders.
简介
从几个方面来看,免疫学的确是一门较难学习的学科。首先,这门学科中具有大量的细节内容,有些时候,这些细节会妨碍你对一些基本概念的理解。 To get around this problem, we're going to concentrate on the big picture. It will be easy for you to find the details somewhere else. Another difficulty in learning immunology is that there is an exception to every rule. Immunologists love these exceptions, because they give clues as to how the immune system functions. But for now, we're just going to learn the rules. Oh sure, we'll come upon exceptions from time to time, but we won't dwell on them. Our goal is to examine the immune system, stripped to its essence.
A third difficulty in studying immunology is that our knowledge of the immune system is still evolving. As you'll see, there are many unanswered questions, and some of the things that seem true today will be proven false tomorrow. I'll try to give you a feeling for the way things stand now, and from time to time I'll discuss what immunologists speculate may be true. But keep in mind that although I'll try to be straight with you, some of the things I'll tell you will change in the future – maybe even by the time you read this!
Although these three features make studying immunology difficult, I think the main reason immunology is such a tough subject is that the immune system is a "team effort" that involves many different players interacting with each other. Imagine you're watching a football game on TV, and the camera is isolated on one player, say, the tight end. You see him run at full speed down the field, and then stop. It doesn't seem to make any sense. Later, however, you see the same play on the big screen, and now you understand. That tight end took two defenders with him down the field, leaving the running back uncovered to catch the pass and run for a touchdown. The immune system is a lot like a football team. It's a network of players who cooperate to get things done, and focusing on a single player doesn't make much sense. You need an overall view. That's the purpose of this first lecture, which you might call "turbo immunology." Here, I'm going to take you on a quick tour of the immune system, so you can get a feeling for how it all fits together. Then in the next lectures, we'll go back and take a closer look at the individual players and their interactions.
物理屏障
Our first line of defense against invaders consists of physical barriers, and to cause real trouble viruses, bacteria, parasites, and fungi must penetrate these shields. Although we tend to think of our skin as the main barrier, the area covered by our skin is only about 2 square meters. In contrast, the area covered by the mucous membranes that line our digestive, respiratory, and reproductive tracts measures about 400 square meters – an area about as big as two tennis courts. The main point here is that there is a large perimeter which must be defended.
先天免疫系统
Any invader that breaches the physical barrier of skin or mucosa is greeted by the innate immune system – our second line of defense. Immunologists call this system "innate" because it is a defense that all animals just naturally seem to have. Indeed, some of the weapons of the innate immune system have been around for more than 500 million years. Let me give you an example of how this amazing innate system works.
Imagine you are getting out of your hot tub, and as you step onto the deck, you get a large splinter in your big toe. On that splinter are many bacteria, and within a few hours you'll notice (unless you had a lot to drink in that hot tub!) that the area around where the splinter entered is red and swollen. These are indications that your innate immune system has kicked in. Your tissues are home to roving bands of white blood cells that defend you against attack. To us, tissue looks pretty solid, but that's because we're so big. To a cell, tissue looks somewhat like a sponge with holes through which individual cells can move rather freely. One of the defender cells that is stationed in your tissues is the most famous innate immune system player of them all: the macrophage. If you are a bacterium, a macrophage is the last cell you want to meet after your ride on that splinter! Here is an electron micrograph showing a macrophage about to devour a bacterium.
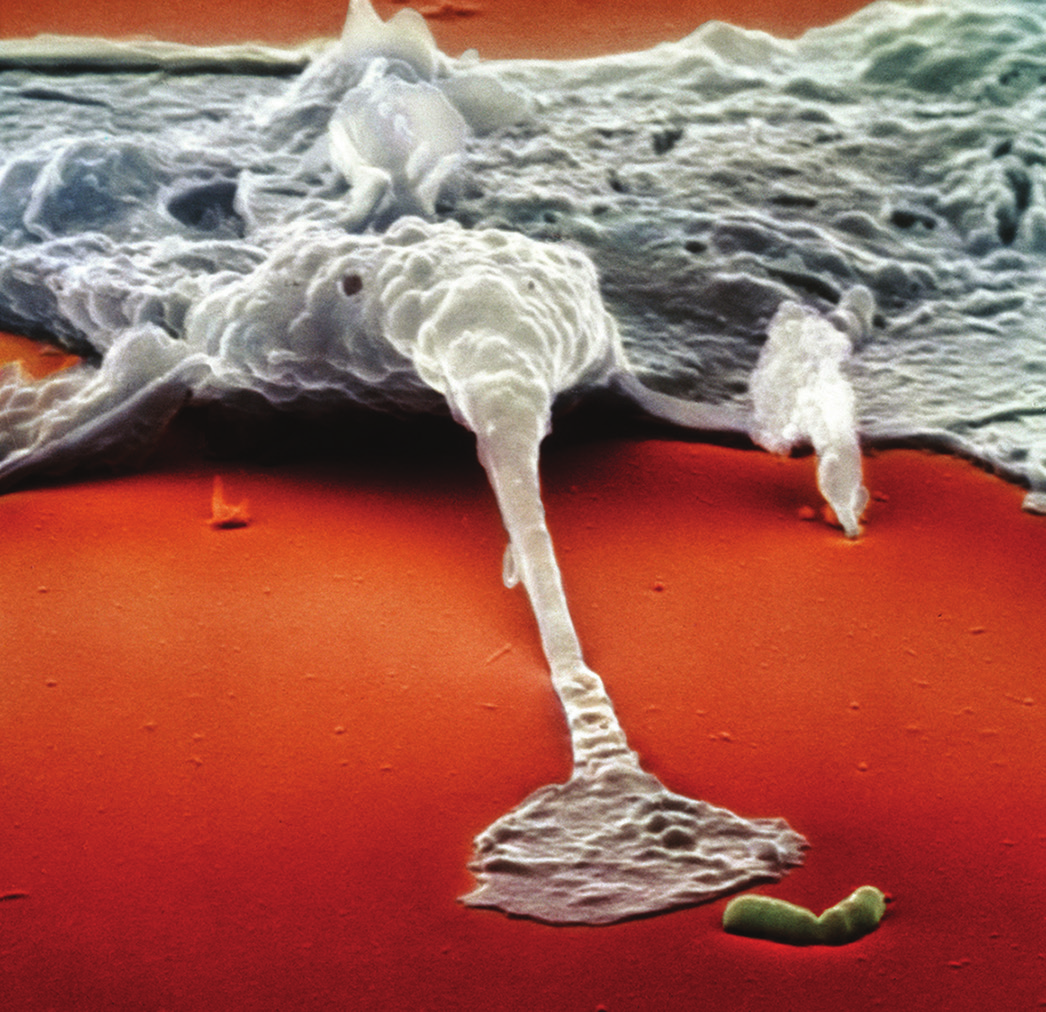
You will notice that this macrophage isn't just waiting until it bumps into the bacterium purely by chance. No, this macrophage actually has sensed the presence of the bacterium and is reaching out a "foot" to grab it. But how does a macrophage know that a bacterium is out there? The answer is that macrophages have antennae (receptors) on their surface which are tuned to recognize "danger molecules" characteristic of common microbial invaders. For example, the membranes that surround bacteria are made up of certain fats and carbohydrates that normally are not found in the human body. Some of these foreign molecules represent "find me and eat me" signals for macrophages. And when macrophages detect danger molecules, they begin to crawl toward the microbe that is emitting these molecules.
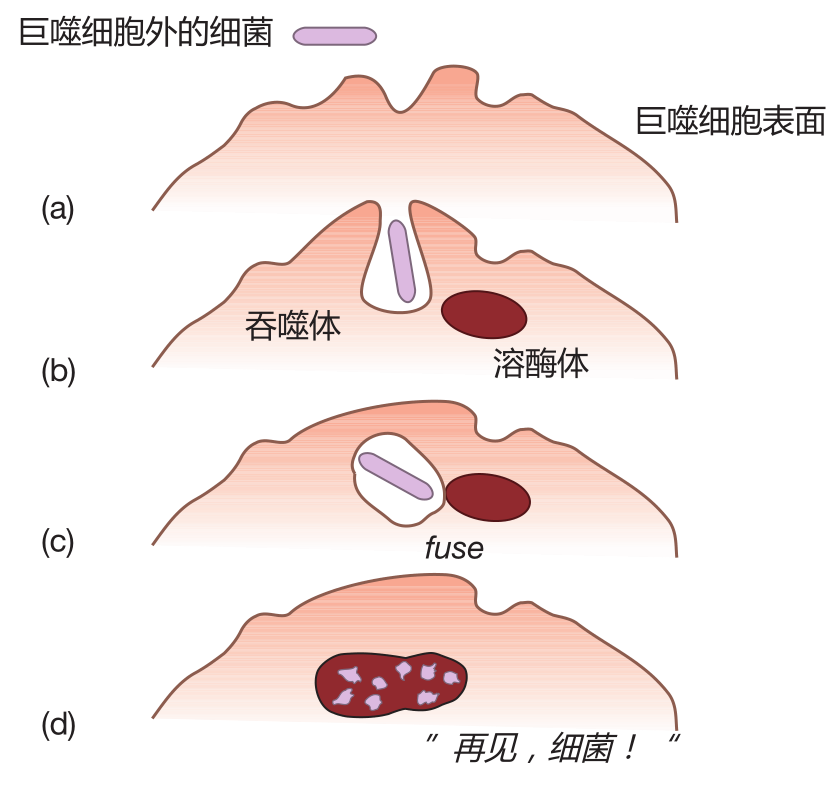
When it encounters a bacterium, a macrophage first engulfs it in a pouch (vesicle) called a phagosome. The vesicle containing the bacterium is then taken inside the macrophage, where it fuses with another vesicle termed a lysosome. Lysosomes contain powerful chemicals and enzymes which can destroy bacteria. In fact, these agents are so destructive that they would kill the macrophage itself if they were released inside it. That's why they are confined within vesicles. Using this clever strategy, the macrophage can destroy an invader without "shooting itself in the foot." This whole process is called phagocytosis, and this series of snapshots shows how it happens.
Macrophages have been around for a very long time. In fact, the ingestion technique macrophages employ is simply a refinement of the strategy that amoebas use to feed themselves – and amoebas have roamed Earth for about 2.5 billion years. So why is this creature called a macrophage? "Macro," of course, means large – and a macrophage is a large cell. Phage comes from a Greek word meaning "to eat." So a macrophage is a big eater. In fact, in addition to defending against invaders, the macrophage also functions as a garbage collector. It will eat almost anything. Immunologists can take advantage of this appetite by feeding macrophages iron filings. Then, using a small magnet, they can separate macrophages from other cells in a cell mixture. Really!
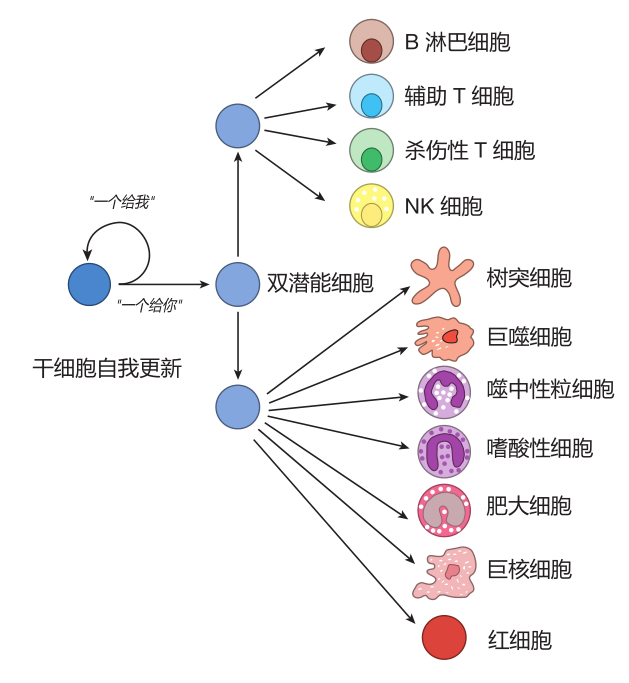
Where do macrophages come from? Macrophages and all the other blood cells in your body are the descendants of self-renewing blood stem cells – the cells from which all the blood cells "stem." By self-renewing, I mean that when a stem cell grows and divides into two daughter cells, it does a "one for me, one for you" thing in which some of the daughter cells go back to being stem cells, and some of the daughters go on to become mature blood cells. This strategy of continual self-renewal insures that there will always be blood stem cells in reserve to carry on the process of making mature blood cells.
Macrophages are so important to our defense that they actually take up their sentinel positions in the tissues well before we are born. After birth, blood stem cells, which reside in the bone marrow, can replenish the supply of macrophages and all the other blood cells as they are needed. As the daughters of blood stem cells mature, they must make choices that determine which type of blood cell they will become when they grow up. As you can imagine, these choices are not random, but are carefully controlled to make sure you have enough of each kind of blood cell. For example, some daughter cells become red blood cells, which capture oxygen in the lungs and transport it to all parts of the body. Our stem cell "factories" must turn out more than two million new red blood cells each second to replace those lost due to normal wear and tear. Other descendants of a blood stem cell may become macrophages, neutrophils, or other types of "white" blood cells. And just as white wine really isn't white, these cells aren't white either. They are colorless, but biologists use the term "white" to indicate that they lack hemoglobin, and therefore are not red. Here is a figure showing some of the many different kinds of blood cells a stem cell can become.
When the cells that can mature into macrophages first exit the bone marrow and enter the blood stream, they are called monocytes. All in all, you have about two billion of these cells circulating in your blood at any one time. This may seem a little creepy, but you can be very glad they are there. Without them, you'd be in deep trouble. Monocytes remain in the blood for an average of about three days. During this time they travel to the capillaries – which represent the "end of the line" for blood vessels – looking for a crack between the endothelial cells that line the inside of the capillaries. These endothelial cells are shaped like shingles, and by sticking a foot between them a monocyte can leave the blood, enter the tissues, and mature into a macrophage. In the tissues, most macrophages just hang out, do their garbage collecting thing, and wait for you to get that splinter so they can do some real work.
When macrophages eat the bacteria on that splinter in your foot, they give off chemicals which increase the flow of blood to the vicinity of the wound. The buildup of blood in this area is what makes your toe red. Some of these chemicals also cause the cells that line the blood vessels to contract, leaving spaces between them so that fluid from the capillaries can leak out into the tissues. It is this fluid that causes the swelling. In addition, chemicals released by macrophages can stimulate nerves in the tissues that surround the splinter, sending pain signals to your brain to alert you that something isn't quite right in the area of your big toe.
During their battle with bacteria, macrophages produce and give off (secrete) proteins called cytokines. These are hormone-like messengers which facilitate communication between cells of the immune system. Some of these cytokines alert monocytes and other immune system cells traveling in nearby capillaries that the battle is on, and encourage these cells to exit the blood to help fight the rapidly multiplying bacteria. Pretty soon, you have a vigorous "inflammatory" response going on in your toe, as the innate immune system battles to eliminate the invaders.
So here's the strategy: You have a large perimeter to defend, so you station sentinels (macrophages) to check for invaders. When these sentinels encounter the enemy, they send out signals (cytokines) that recruit more defenders to the site of the battle. The macrophages then do their best to hold off the invaders until reinforcements arrive. Because the innate response involves warriors such as macrophages, which are programmed to recognize many common invaders, your innate immune system usually responds so quickly that the battle is over in just a few days.
There are other players on the innate team. For example, in addition to the professional phagocytes such as macrophages, which make it their business to eat invaders, the innate system also includes the complement proteins that can punch holes in bacteria, and natural killer cells which are able to destroy bacteria, parasites, virus-infected cells, and some cancer cells. We will talk more about the macrophage's innate system teammates in the next lecture.
获得性免疫系统
About 99% of all animals get along just fine with only natural barriers and the innate immune system to protect them. However, vertebrates like us have a third level of defense: the adaptive immune system. This is a defense system which actually can adapt to protect us against almost any invader. One of the first clues that the adaptive immune system existed came back in the 1790s when Edward Jenner began vaccinating the English against smallpox virus. In those days, smallpox was a major health problem. Hundreds of thousands of people died from this disease, and many more were horribly disfigured. What Jenner observed was that milkmaids frequently contracted a disease called cowpox, which caused lesions on their hands that looked similar to the sores caused by the smallpox virus. Jenner also noted that milkmaids who had contracted cowpox almost never got smallpox (which, it turns out, is caused by a close relative of the cowpox virus).
So Jenner decided to conduct a daring experiment. He collected pus from the sores of a milkmaid who had cowpox, and used it to inoculate a little boy named James Phipps. Later, when Phipps was re-inoculated with pus from the sores of a person infected with smallpox, he did not contract that disease. In Latin, the word for cow is vacca – which explains where we get the word vaccine. History makes out the hero in this affair to be Edward Jenner, but I think the real hero that day was the young boy. Imagine having this big man approach you with a large needle and a tube full of pus! Although this isn't the sort of thing that could be done today, we can be thankful that Jenner's experiment was a success, because it paved the way for vaccinations that have saved countless lives.
Smallpox virus was not something humans encountered regularly. So Jenner's experiment showed that if the human immune system was given time to prepare, it could produce weapons that could provide protection against an intruder it had never seen before. Importantly, the smallpox vaccination only protected against small-pox or closely related viruses such as cowpox. James Phipps was still able to get mumps, measles, and the rest. This is one of the hallmarks of the adaptive immune system: It adapts to defend against specific invaders.
抗体和B细胞
Eventually, immunologists determined that immunity to smallpox was conferred by special proteins that circulated in the blood of immunized individuals. These proteins were named antibodies, and the agent that caused the antibodies to be made was called an antigen – in this case, the cowpox virus. Here's a sketch that shows the prototype antibody, immunoglobulin G (IgG).
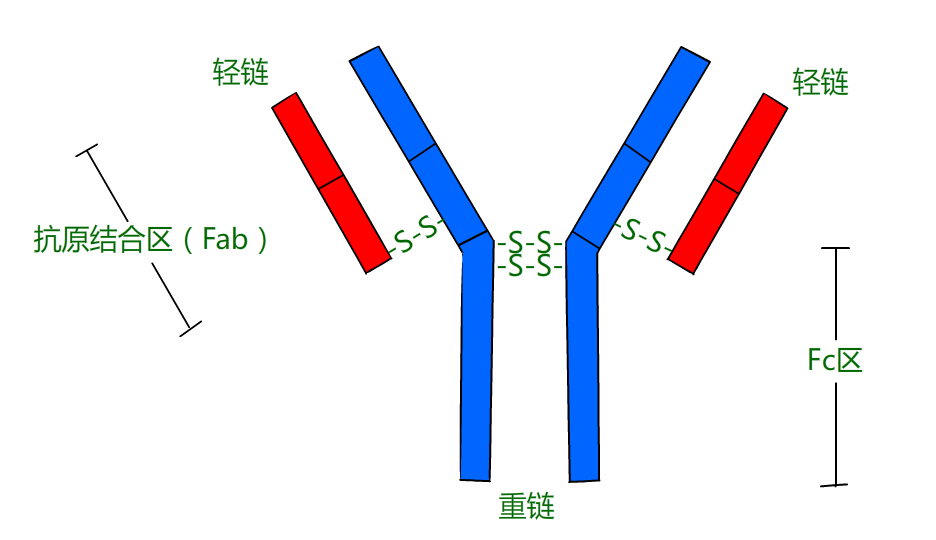
As you can see, an IgG antibody molecule is made up of two pairs of two different proteins, the heavy chain (Hc) and the light chain (Lc). Because of this structure, each molecule has two identical "hands" (Fab regions) that can bind to antigens. Proteins are the ideal molecules to use for constructing antibodies that can grasp attackers because different proteins can fold up into a myriad of complex shapes.
IgG makes up about 75% of the antibodies in the blood, but there are four other classes of antibodies: IgA, IgD, IgE, and IgM. Each kind of antibody is produced by B cells – white blood cells that are born in the bone marrow, and which can mature to become antibody factories called plasma B cells.
In addition to having hands that can bind to an antigen, an antibody molecule also has a constant region (Fc) "tail" which can bind to receptors (Fc receptors) on the surface of cells such as macrophages. In fact, it is the special structure of the antibody Fc region that determines its class (e.g., IgG vs. IgA), which immune system cells it will bind to, and how it will function.
The hands of each antibody bind to a specific antigen (e.g., a protein on the surface of the smallpox virus), so in order to have antibodies available that can bind to many different antigens, many different antibody molecules are required. Now, if we want antibodies to protect us from every possible invader (and we do!), how many different antibodies would we need? Well, immunologists estimate that about 100 million should do the trick. Since each antigen-binding region of an antibody is composed of a heavy chain and a light chain, we could mix and match about 10,000 different heavy chains with 10,000 different light chains to get the 100 million different antibodies we need. However, human cells only have about 25,000 genes in all, so if each heavy or light chain protein were encoded by a different gene, most of a human's genetic information would be used up just to make antibodies. You see the problem.
模块化设计产生抗体多样性
The riddle of how B cells could produce the 100 million different antibodies required to protect us was solved in 1977 by Susumu Tonegawa, who received the Nobel Prize for his discovery. When Tonegawa started working on this problem, the dogma was that the DNA in every cell in the body was the same. This made perfect sense, because after an egg is fertilized, the DNA in the egg is copied. These copies are then passed down to the daughter cells, where they are copied again, and passed down to their daughters – and so on. Therefore, barring errors in copying, each of our cells should end up with the same DNA as the original, fertilized egg. Tonegawa, however, hypothesized that although this is probably true in general, there might be exceptions. His idea was that all of our B cells might start out with the same DNA, but that as these cells mature, the DNA that makes up the antibody genes might change – and these changes might be enough to generate the 100 million different antibodies we need.
Tonegawa decided to test this hypothesis by comparing the DNA sequence of the light chain from a mature B cell with the DNA sequence of the light chain from an immature B cell. Sure enough, he found that they were different, and that they were different in a very interesting way. What Tonegawa and others discovered was that the mature antibody genes are made by modular design.
In every B cell, on the chromosomes that encode the antibody heavy chain there are multiple copies of four types of DNA modules (gene segments) called V, D, J, and C. Each copy of a given module is slightly different from the other copies of that module. For example, in humans there are about 40 different V segments, about 25 different D segments, 6 different J segments, and so on. To assemble a mature heavy chain gene, each B cell chooses (more or less at random) one of each kind of gene segment, and pastes them together like this.
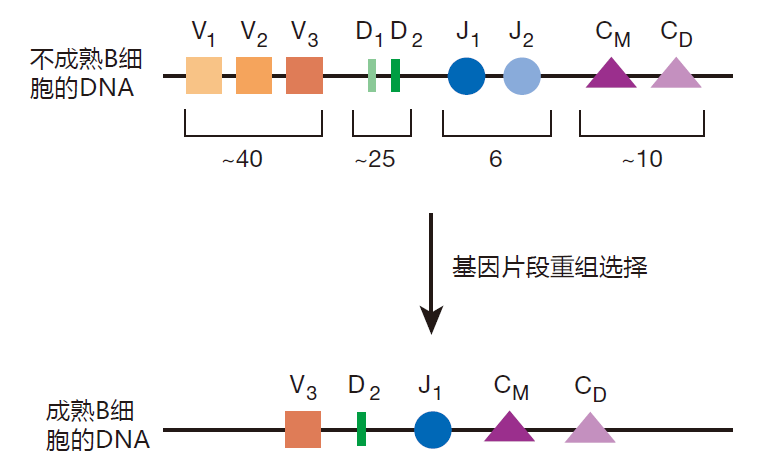
You have seen this kind of mix-and-match strategy used before to create diversity. For example, 20 different amino acids are mixed and matched to create the huge number of different proteins that our cells produce. And to create genetic diversity, the chromosomes you inherited from your mother and father are mixed and matched to make the set of chromosomes that goes into your egg or sperm cells. Once Mother Nature gets a good idea, she uses it over and over – and modular design is one of her very best ideas.
The DNA that encodes the light chain of the antibody molecule is also assembled by picking gene segments and pasting them together. Because there are so many different gene segments that can be mixed and matched, this scheme can be used to create about 10 million different antibodies – not quite enough. So, to make things even more diverse, when the gene segments are joined together, additional DNA bases are added or deleted. When this junctional diversity is included, there is no problem creating 100 million B cells, each with the ability to make a different antibody. The magic of this scheme is that by using modular design and junctional diversity, only a small amount of genetic information is required to create incredible antibody diversity.
克隆选择
In the human blood stream there is a total of about three billion B cells. This seems like a lot, but if there are 100 million different kinds of B cells (to produce the 100 million different kinds of antibodies we need for protection), this means that, on average, there will only be about 30 B cells in the blood that can produce an antibody which will bind to a given antigen (e.g., a protein on the surface of a virus). Said another way, although we have B cells in our arsenal that can deal with essentially any invader, we don't have a lot of any one kind of B cell. As a result, when we are attacked, more of the appropriate B cells must be made. Indeed, B cells are made "on demand." But how does the immune system know which B cells to make more of? The solution to this problem is one of the most elegant in all of immunology: the principle of clonal selection.
After B cells do their mix-and-match thing and paste together the modules required to form the "recipes" for their heavy and light chain antibody proteins, a relatively small number of these proteins is made – a "test batch" of antibody molecules, if you will. These tester antibodies, called B cell receptors (BCRs), are transported to the surface of the B cell and are tethered there with their antigen-binding regions facing out. Each B cell has roughly 100,000 BCRs anchored on its surface, and all the BCRs on a given B cell recognize the same antigen.
The B cell receptors on the surface of a B cell act like "bait." What they are "fishing for" is the molecule which their Fab regions have the right shape to grasp – their cognate antigen. Sadly, the vast majority of B cells fish in vain. For example, most of us will never be infected with the SARS virus or the AIDS virus. Consequently, those B cells in our body which could make antibodies that recognize these viruses never will find their match. It must be very frustrating for most B cells. They fish all their lives, and never catch anything!
On occasion, however, a B cell does make a catch. And when a B cell's receptors bind to its cognate antigen, that B cell is triggered to double in size and divide into two daughter cells – a process immunologists call proliferation. Both daughter cells then double in size and divide to produce a total of four cells, and so forth. Each cycle of cell growth and division takes about 12 hours to complete, and this period of proliferation usually lasts about a week. At the end of this time, a "clone" of roughly 20,000 identical B cells will have been produced, all of which have receptors on their surface that can recognize the same antigen. Now there are enough B cells to mount a real defense!
After the selected B cells proliferate to form this large clone, most of them begin to make antibodies in earnest. The antibodies produced by these selected B cells are slightly different from the antibody molecules displayed on their surface in that there is no "anchor" to attach them to the B cell's surface. As a result, these antibodies are transported out of the B cell and into the blood stream. One B cell, working at full capacity, can pump out about 2,000 antibody molecules per second! After making this heroic effort, most of these B cells die, having worked for only about a week as antibody factories.
When you think about it, this is a marvelous strategy. First, because they employ modular design, B cells use relatively few genes to create enough different antibody molecules to recognize any possible invader. Second, B cells are made on demand. So instead of filling up our bodies with a huge number of B cells which may never be used, we begin with a relatively small number of B cells, and then select the particular B cells that will be useful against the "invader du jour." Once selected, the B cells proliferate rapidly to produce a large clone of B cells whose antibodies are guaranteed to be useful against the invader. Third, after the clone of B cells has grown sufficiently large, most of these cells become antibody factories which manufacture huge quantities of the very antibodies that are right to defend against the invader. Finally, when the intruder has been conquered, most of the B cells die. As a result, we don't fill up with B cells that are appropriate to defend against yesterday's invader, but which would be useless against the enemy that attacks us tomorrow. I love this system!
抗体的功能
Interestingly, although antibodies are very important in the defense against invaders, they really don't kill anything. Their job is to plant the "kiss of death" on an invader – to tag it for destruction. If you go to a fancy wedding, you'll usually pass through a receiving line before you are allowed to enjoy the champagne and cake. Of course, one of the functions of this receiving line is to introduce everyone to the bride and groom. But the other function is to be sure no outsiders are admitted to the celebration. As you pass through the line, you will be screened by someone who is familiar with all the invited guests. If she finds that you don't belong there, she will call the bouncer and have you removed. She doesn't do it herself – certainly not. Her role is to identify undesirables, not to show them to the door. And it's the same with antibodies: They identify invaders, and let other players do the dirty work.
In developed countries, the invaders we encounter most frequently are bacteria and viruses. Antibodies can bind to both types of invaders and tag them for destruction. Immunologists like to say that antibodies can opsonize these invaders. This term comes from a German word that means "to prepare for eating." I like to equate opsonize with "decorate," because I picture these bacteria and viruses with antibodies hanging all over them, decorating their surfaces. Anyway, when antibodies opsonize bacteria or viruses, they do so by binding to the invader with their Fab regions, leaving their Fc tails available to bind to Fc receptors on the surface of cells such as macrophages. Using this strategy, antibodies can form a bridge between the invader and the phagocyte, bringing the invader in close, and preparing it for phagocytosis.
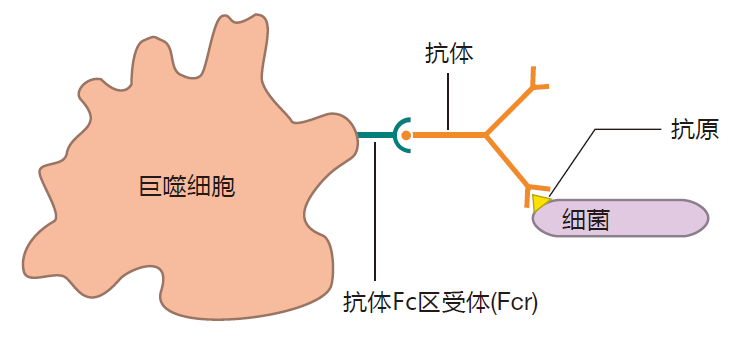
In fact, it's even better than this. When a phagocyte's Fc receptors bind to antibodies that are opsonizing an invader, the appetite of the phagocyte increases, making it even more phagocytic. Macrophages have proteins on their surface that can bind directly to many common invaders. However, the ability of antibodies to form a bridge between a macrophage and an invader allows a macrophage to increase its catalog of enemies to include any invader to which an antibody can bind, common or uncommon. In effect, antibodies focus a macrophage's attention on invaders, some of which (the uncommon ones) a macrophage would otherwise ignore.
During a viral attack, antibodies can do something else that is very important. Viruses enter our cells by binding to certain receptor molecules on a cell's surface. Of course these receptors are not placed there for the convenience of the virus. They are normal receptors, such as the Fc receptor, that have quite legitimate functions, but which the virus has learned to use to its own advantage. Once it has bound to these receptors and entered a cell, a virus then uses the cell's machinery to make many copies of itself. These newly made viruses burst out of the cell, sometimes killing it, and go on to infect neighboring cells. Now for the neat part: Antibodies can actually bind to a virus while it is still outside of a cell, and can keep the virus either from entering the cell or from reproducing once it has entered. Antibodies with these special properties are called neutralizing antibodies. For example, some neutralizing antibodies can prevent a virus from "docking" on the surface of a cell by binding to the part of the virus that normally would plug into the cellular receptor. When this happens, the virus is "hung out to dry," opsonized and ready to be eaten by phagocytes!
T细胞
Although antibodies can tag viruses for phagocytic ingestion, and can help keep viruses from infecting cells, there is a weakness in the antibody defense against viruses: Once a virus gets into a cell, antibodies can't get to it, so the virus is safe to make thousands of copies of itself. To deal with this potential problem, the immune system evolved to include another weapon: the killer T cell, which is another member of the adaptive immune system team.
The importance of T cells is suggested by the fact that an adult human has about 300 billion of them. T cells are very similar to B cells in appearance. In fact, under an ordinary microscope, an immunologist can't tell them apart. Like B cells, T cells are produced in the bone marrow, and on their surface they display antibody-like molecules called T cell receptors (TCRs). Like the B cell's receptors (the antibody molecules attached to its surface), TCRs also are made by a mix-and-match, modular design strategy. As a result, TCRs are about as diverse as BCRs. T cells also employ the principle of clonal selection: When a T cell's receptors bind to their cognate antigen, the T cell proliferates to build up a clone of T cells with the same specificity. This proliferation stage takes about a week to complete, so just like the antibody response, the T cell response is slow and specific.
Although they are similar in many ways, there are also important differences between B cells and T cells. Whereas B cells mature in the bone marrow, T cells mature in the thymus (that's why they're called "T" cells). Further, whereas B cells make antibodies that can recognize any organic molecule, T cells specialize in recognizing protein antigens. In addition, a B cell can secrete its receptors in the form of antibodies, but a T cell's receptors remain tightly glued to its surface. Perhaps most importantly, a B cell can recognize an antigen "by itself," whereas a T cell will only recognize an antigen if it is "properly presented" by another cell. I'll explain what that means in a bit.
There are actually three main types of T cells: killer T cells (frequently called cytotoxic lymphocytes or CTLs), helper T cells, and regulatory T cells. The killer T cell is a potent weapon that can destroy virus-infected cells. Indeed, by recognizing and killing these cells, the CTL solves the "hiding virus" problem – the weakness I mentioned in the antibody defense against viruses. The way a killer T cell destroys virus-infected cells is by making contact with its target and then triggering the cell to commit suicide! This "assisted suicide" is a great way to deal with viruses that have infected cells, because when a virus-infected cell dies, the viruses within the cell die also.
The second type of T cell is the helper T cell (Th cell). As you will see, this cell serves as the quarterback of the immune system team. It directs the action by secreting chemical messengers (cytokines) that have dramatic effects on other immune system cells. These cytokines have names like interleukin 2 (IL-2) and interferon gamma (IFN-γ), and we will discuss what they do in later lectures. For now, it is only important to realize that helper T cells are basically cytokine factories.
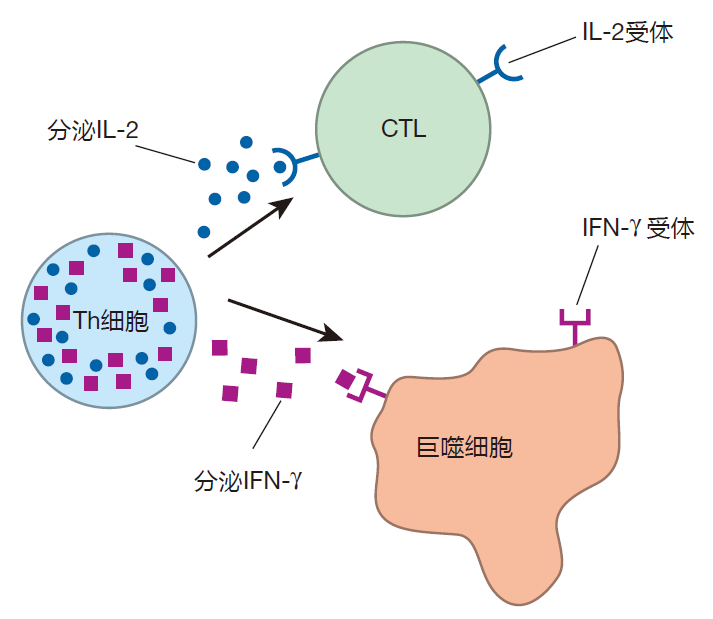
The third type of T cell is the regulatory T cell (Treg). The role of this type of T cell is to keep the immune system from overreacting or from reacting inappropriately. Immunologists are still working to understand how T cells become regulatory T cells, and exactly how Tregs perform these important functions.
抗原的提呈
One thing I need to clear up is exactly how antigen is presented to T cells. It turns out that special proteins called major histocompatibility complex (MHC) proteins do the "presenting," and that T cells use their receptors to "view" this presented antigen. As you may know, "histo" means tissue, and these major histocompatibility proteins, in addition to being presentation molecules, are also involved in the rejection of transplanted organs. In fact, when you hear that someone is waiting for a "matched" kidney, it's the MHC molecules of the donor and the recipient that the transplant surgeon is trying to match.
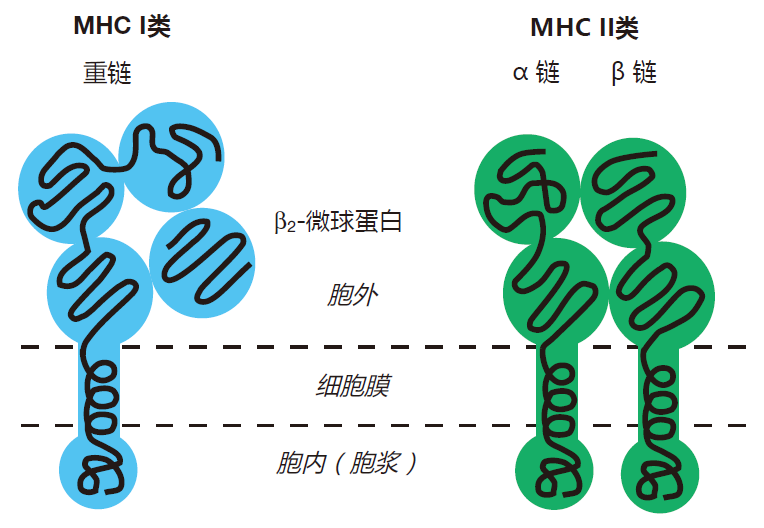
There are two types of MHC molecules, called class I and class II. Class I MHC molecules are found in varying amounts on the surface of most cells in the body. Class I MHC molecules function as "billboards," which inform killer T cells about what is going on inside these cells. For example, when a human cell is infected by a virus, fragments of viral proteins called peptides are loaded onto class I MHC molecules, and transported to the surface of the infected cell. By inspecting these protein fragments displayed by class I MHC molecules, killer T cells can use their receptors to "look into" the cell to discover that it has been infected and that it should be destroyed.
Class II MHC molecules also function as billboards, but this display is intended for the enlightenment of helper T cells. Only certain cells in the body make class II MHC molecules, and these are called antigen presenting cells (APCs). Macrophages, for example, are excellent antigen presenting cells. During a bacterial infection, a macrophage will "eat" bacteria, and will load fragments of ingested bacterial proteins onto class II MHC molecules for display on the surface of the macrophage. Then, using their T cell receptors, helper T cells can scan the macrophage's class II MHC billboards for news of the bacterial infection. So class I MHC molecules alert killer T cells when something isn't right inside a cell, and class II MHC molecules displayed on APCs inform helper T cells that problems exist outside of cells.
Although a class I MHC molecule is made up of one long chain (the heavy chain) plus a short chain (β2-microglobulin), and a class II MHC molecule has two long chains (α and β), you'll notice that these molecules are rather similar in appearance.
Okay, I know it's hard to visualize the real shapes of molecules from drawings like this, so I thought I'd show you a few pictures that may make this more real. Here's what an empty MHC molecule might look like from the viewpoint of the T cell receptor. Right away you see the groove into which the protein fragment would fit.

Next, let's look at a fully-loaded, class I molecule.

I can tell it's a class I MHC molecule because the peptide is contained nicely within the groove. It turns out that the ends of the groove of a class I molecule are closed, so a protein fragment must be about nine amino acids in length to fit in properly. Class II MHC molecules are slightly different.

Here you see that the peptide overflows the groove. This works fine for class II, because the ends of the groove are open, so protein fragments as large as about 20 amino acids fit nicely.
So MHC molecules resemble buns, and the protein fragments they present resemble wieners. And if you imagine that the cells in our bodies have hot dogs on their surfaces, you won't be far wrong about antigen presentation. That's certainly the way I picture it!
获得性免疫系统的激活
Because B and T cells are such potent weapons, there is a requirement that cells of the adaptive immune system must be activated before they can function. Collectively, B and T cells are called lymphocytes, and how they are activated is one of the key issues in immunology. To introduce this concept, I will sketch how helper T cells are activated.
The first step in the activation of a helper T cell is recognition of its cognate antigen (e.g., a fragment of a bacterial protein) displayed by class II MHC molecules on the surface of an antigen presenting cell. But seeing its cognate antigen on that billboard isn't enough – a second signal or "key" also is required for activation. This second signal is non-specific (it's the same for any antigen), and it involves a protein (B7 in this drawing) on the surface of an antigen presenting cell that plugs into its receptor (CD28 in this drawing) on the surface of the helper T cell.
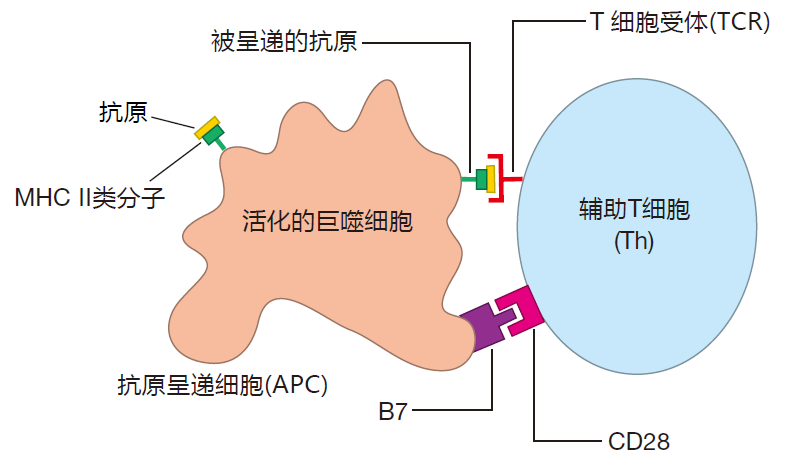
You see an example of this kind of two-key system when you visit your safe deposit box. You bring with you a key that is specific for your box – it won't fit any other. The bank teller provides a second, non-specific key that will fit all the boxes. Only when both keys are inserted into the locks on your box can it be opened. Your specific key alone won't do it, and the teller's non-specific key alone won't either. You need both. Now, why do you suppose helper T cells and other cells of the adaptive immune system require two keys for activation? For safety, of course – just like your bank box. These cells are powerful weapons that must only be activated at the appropriate time and place.
Once a helper T cell has been activated by this two-key system, it proliferates to build up a clone composed of many helper T cells whose receptors recognize the same antigen. These helper cells then mature into cells that can produce the cytokines needed to direct the activities of the immune system. B cells and killer T cells also require two-key systems for their activation, and we'll talk about them in another lecture.
次级淋巴器官
If you've been thinking about how the adaptive immune system might get turned on during an attack, you've probably begun to wonder whether this could ever happen. After all, there are only between 100 and 1,000 T cells that will have TCRs specific for a given invader, and for these T cells to be activated, they must come in contact with an antigen presenting cell that has "seen" that invader. Given that these T cells and APCs are spread all over the body, it would seem very unlikely that this would happen before an invasion got completely out of hand. Fortunately, to make this work with reasonable probability, the immune system includes "meeting places" – the secondary lymphoid organs. The best known secondary lymphoid organ is the lymph node.
You may not be familiar with the lymphatic system, so I'd better say a few words about it. In your home, you have two plumbing systems. The first supplies the water that comes out of your faucets. This is a pressurized system, with the pressure being provided by a pump. You have another plumbing system that includes the drains in your sinks, showers, and toilets. This second system is not under pressure – the water just flows down the drain and out into the sewer. The two systems are connected in the sense that eventually the wastewater is recycled and used again.
The plumbing in a human is very much like this. We have a pressurized system (the cardiovascular system) in which blood is pumped around the body by the heart. Everybody knows about this one. But we also have another plumbing system – the lymphatic system. This system is not under pressure, and it drains the fluid (lymph) that leaks out of our blood vessels into our tissues. Without this system, our tissues would fill up with fluid and we'd look like the Pillsbury Doughboy. Lymph is collected from the tissues of our lower body into lymphatic vessels, and is transported by these vessels, under the influence of muscular contraction, through a series of one-way valves to the upper torso. This lymph, plus lymph from the left side of the upper torso, is collected into the thoracic duct and emptied into the left subclavian vein to be recycled back into the blood. Likewise, lymph from the right side of the upper body is collected into the right lymphatic duct and is emptied into the right subclavian vein. From this diagram, you can see that as the lymph winds its way back to be reunited with the blood, it passes through a series of way stations – the lymph nodes.
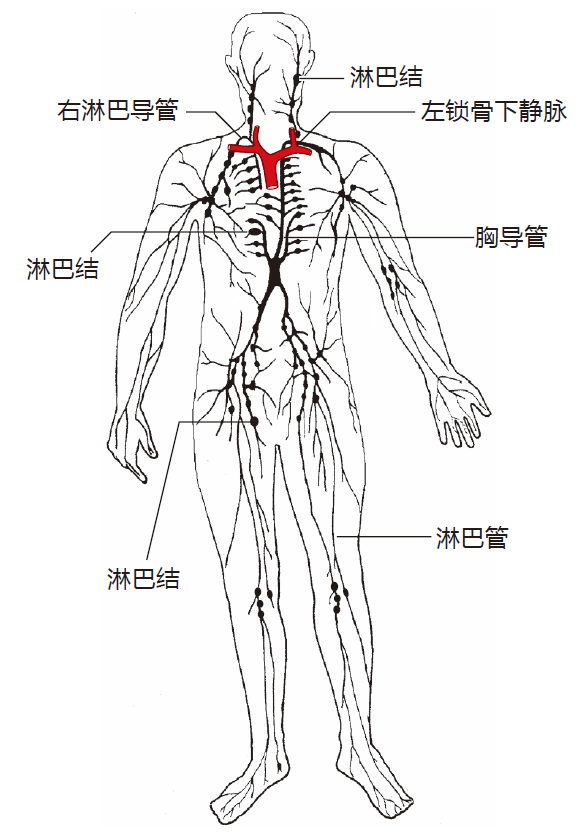
In a human, there are about 500 lymph nodes, ranging in size from very small to almost as big as a Brussels sprout. Most are arrayed in "chains" that are connected by lymphatic vessels. Invaders such as bacteria and viruses are carried by the lymph to nearby nodes, and antigen presenting cells that have picked up foreign antigens in the tissues travel to lymph nodes to present their cargo.
Meanwhile, B cells and T cells circulate from node to node, looking for the antigens for which they are "fated."
So lymph nodes really function as "dating bars" – places where T cells, B cells, APCs, and antigen all gather for the purpose of communication and activation. Bringing these cells and antigens together within the small volume of a lymph node greatly increases the probability that they will interact and efficiently activate the adaptive immune system.
免疫记忆
After B and T cells have been activated, have proliferated to build up clones of cells with identical antigen specificities, and have vanquished the enemy, most of them die off. This is a good thing, because we wouldn't want our immune systems to fill up with old B and T cells. On the other hand, it would be nice if some of these experienced B and T cells would stick around, just in case we are exposed to the same invaders again. That way, the adaptive immune system wouldn't have to start from scratch. And that's just the way it works. These "leftover" B and T cells are called memory cells. In addition to being more numerous than the original, inexperienced B and T cells, memory cells are easier to activate. As a result of this immunological memory, during a second attack, the adaptive system usually can spring into action so quickly that you never even experience any symptoms.
自身耐受
As I mentioned earlier, B cell receptors and T cell receptors are so diverse that they should be able to recognize any invader. However, this diversity poses a potential problem: If B and T cell receptors are this diverse, many of them are certain to recognize our own "self" molecules (e.g., the molecules that make up our cells, or proteins like insulin that circulate in our blood). If this were to happen, our adaptive immune system might attack our own bodies, and we could die from autoimmune disease. Fortunately, B cells and T cells are "screened" to avoid autoimmunity. Although immunologists still don't understand the details of the tests used to eliminate self-reactive B and T cells, this testing is sufficiently rigorous that autoimmune disease is relatively rare.
先天性免疫和获得性免疫的比较
Now that you have met some of the main players, I want to emphasize the differences between the innate and adaptive immune system "teams." Understanding how they differ is crucial to understanding how the immune system works.
Imagine that you are in the middle of town and someone steals your shoes. You look around for a store where you can buy another pair, and the first store you see is called Charlie's Custom Shoes. This store has shoes of every style, color, and size, and the salesperson is able to fit you in exactly the shoes you need. However, when it comes time to pay, you are told that you must wait a week or two to get your shoes – they will have to be custom-made for you, and that will take a while. But you need shoes right now! So they send you across the street to Freddie's Fast Fit – a store that only carries a few styles and sizes. Freddie's wouldn't be able to fit Shaquille O'Neal, but this store does stock shoes in the common sizes that fit most people. Consequently, you can buy a pair of shoes from Freddie's that will tide you over until your custom shoes are made for you.
This is very similar to the way the innate and adaptive immune systems work. The players of the innate system (such as the macrophage) are already in place, and are ready to defend against a relatively small attack by invaders we are likely to meet on a day-to-day basis. Indeed, in many instances, the innate system is so effective and so fast that the adaptive immune system never even kicks in. In other cases, the innate system may be insufficient to deal with an invasion, and the adaptive system will need to be mobilized. This takes time, however, because the B and T cells of the adaptive system must be custom-made through the process of clonal selection and proliferation. Consequently, while these "designer cells" are being produced, the innate immune system must do its best to hold the invaders at bay.
先天性免疫的规律
Immunologists used to believe that the only function of the innate system was to provide a rapid defense which would deal with invaders while the adaptive immune system was getting cranked up. However, it is now clear that the innate system does much more than that.
The adaptive immune system's antigen receptors (BCRs and TCRs) are so diverse that they can probably recognize any protein molecule in the universe. However, the adaptive system is clueless as to which of these molecules is dangerous and which is not. So how does the adaptive system distinguish friend from foe? The answer is that it relies on the judgment of the innate system.
The receptors of the innate system are precisely tuned to detect the presence of the common pathogens (disease-causing agents) we encounter in daily life – viruses, bacteria, fungi, and parasites. In addition, the innate system has receptors that can detect when "uncommon" pathogens kill human cells. Consequently, it is the innate system which is responsible for evaluating the danger and for activating the adaptive immune system. In a real sense, the innate system gives "permission" to the adaptive system to respond to an invasion. But it's even better than that, because the innate system does more than just turn the adaptive system on. The innate system actually integrates all the information it collects about an invader and formulates a plan of action. This "game plan," which the innate system delivers to the adaptive immune system, tells which weapons must be mobilized (e.g., B cells or killer T cells) and exactly where in the body these weapons should be deployed. So if we think of the helper T cell as the quarterback of the adaptive immune system team, we should consider the innate immune system to be the "coach" – for it is the innate system that "scouts" the opponents, designs the game plan, and sends in the plays for the quarter-back to call.
结束语
We have come to the end of our turbo overview of the immune system, and by now you should have a rough idea of how the system works. In the next nine lectures, we will focus more sharply on the individual players of the innate and adaptive system teams, paying special attention to how and where these players interact with each other to make the system function efficiently.


