第13讲 Immunodefciency
HEADS UP!
Because the immune system is highly interconnected, a genetic defect which cripples one of the players can have a major effect on the functioning of the overall system. Moreover, drugs or illnesses that weaken the immune system can leave us vulnerable to infections – infections which would not be a problem for an immune system operating at full strength.
INTRODUCTION
Serious disease may result when our immune system is compromised. Some immunodeficiencies are caused by genetic defects that disable parts of the immune network.
Others are "acquired" as the consequence of malnutrition, deliberate immunosuppression (e.g., during organ transplantation or chemotherapy for cancer), or disease (e.g., AIDS).
GENETIC DEFECTS LEADING TO IMMUNODEFICIENCY
A genetic defect, in which a single gene is mutated, can lead to immune system weakness. For example, individuals who are born with non-functional CD40 or CD40L proteins are unable to mount a T cell-dependent antibody response – because T cells either cannot deliver or B cells cannot receive this all-important, co-stimulatory signal.
Both class switching and somatic hypermutation usually require co-stimulation by CD40L, so one result of the CD40–CD40L defect is that B cells secrete mainly IgM antibodies which have not affinity matured. Other genetic deficiencies affect the formation of the thymus. In one such disease, DiGeorge syndrome, essentially all thymic tissue is missing.
People with this disorder are susceptible to life-threatening infections because they lack functional T cells.
Genetic defects also can knock out both B and T cells.
This group of diseases is called severe combined immunodeficiency syndrome (SCIDS) – where the "combined" label indicates that neither B nor T cells function properly.
It was this disease that forced David Vetter, the famous "bubble boy," to live for 12 years in a pathogen-free, plastic bubble. Although a number of different mutations can result in SCIDS, the best-studied mutation causes a defect in a protein that initiates the gene splicing required to produce B and T cell receptors. Without their receptors, B and T cells are totally useless.
Immunodeficiencies also can result from genetic defects in the innate immune system. For example, people who are born with mutations in important complement proteins (e.g., C3) have lymph nodes with no germinal centers and B cells that produce mainly IgM antibodies.
Given the large number of different proteins involved in making the innate and adaptive immune systems work effectively, it's pretty amazing that mutations leading to immunodeficiency are so rare. In fact, inherited immunodeficiencies affect only about one in 10,000 newborns. It is likely, however, that many other cases of genetic immunodefi ciency go undetected because our functionally redundant immune system has evolved to provide "backups" when elements of the main system are disabled.
AIDS
Although genetic immunodeficiencies are relatively rare, millions of people suffer from immunodeficiencies that are acquired. A large group of immunodeficient humans acquired their deficiency when they were infected with the AIDS virus – a virus that currently infects about 40 million people worldwide, and has resulted in more than 30 million deaths. The AIDS
symptoms which originally alerted physicians that they were dealing with a disease which had immunodeficiency as its basis were the high incidence of infections (e.g., Pneumocystis carinii pneumonia) or cancers (e.g., Kaposi sarcoma) that usually were seen only in immunosuppressed individuals. Soon, the virus that caused this immunodeficiency was isolated and named the human immunodeficiency virus number one (HIV-1).
Currently, this is the world's most intensely studied virus, with nearly a billion dollars being spent annually to try to discover its secrets.
An HIV-1 infection
The early events in a human HIV-1 infection are not well characterized because the infection typically is not diagnosed until weeks or months after exposure to the virus.
However, the emerging picture is that these infections typically begin when the virus penetrates the rectal or vaginal mucosa and infects helper T cells which lie below these protective surfaces. The virus uses these cells' biosynthetic machinery to make many more copies of itself, and the newly made viruses then infect other cells. So in the early stages of infection, the virus multiplies relatively unchecked while the innate system gives it its best shot, and the adaptive system is being mobilized. After a week or so, the adaptive system starts to kick in, and virus-specific B cells, helper T cells, and CTLs are activated, proliferate, and begin to do their thing. During this early, acute phase of the infection, there is a dramatic rise in the number of viruses in the body (the viral load) as the virus multiplies in infected cells. The viral load peaks three to four weeks post infection, and this peak is followed by a marked decrease in the viral load as virus-specific CTLs go to work.
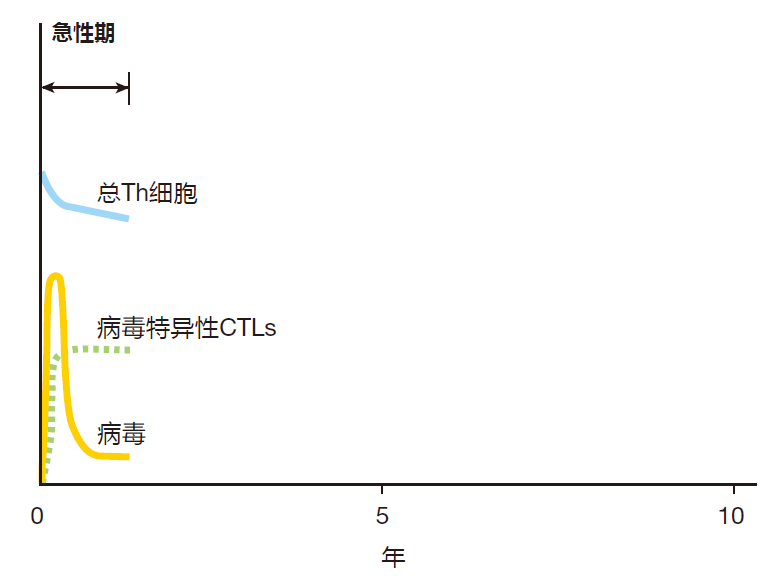
With many viruses (e.g., smallpox), the end result of the acute phase of a viral infection is sterilization: The immune system destroys all the invading viruses, and memory B and T cells are produced to protect against a subsequent infection by the same virus. In contrast, a full-blown HIV-1 infection always leads to a chronic phase that can last for ten or more years. During this phase, a fierce struggle goes on between the immune system and the AIDS virus – a battle which the virus almost always wins.
During the chronic phase of infection, viral loads decrease to low levels compared with those reached during the height of the acute phase, but the number of virus-specific CTLs and Th cells remains high – a sign that the immune system is still trying hard to defeat the virus.
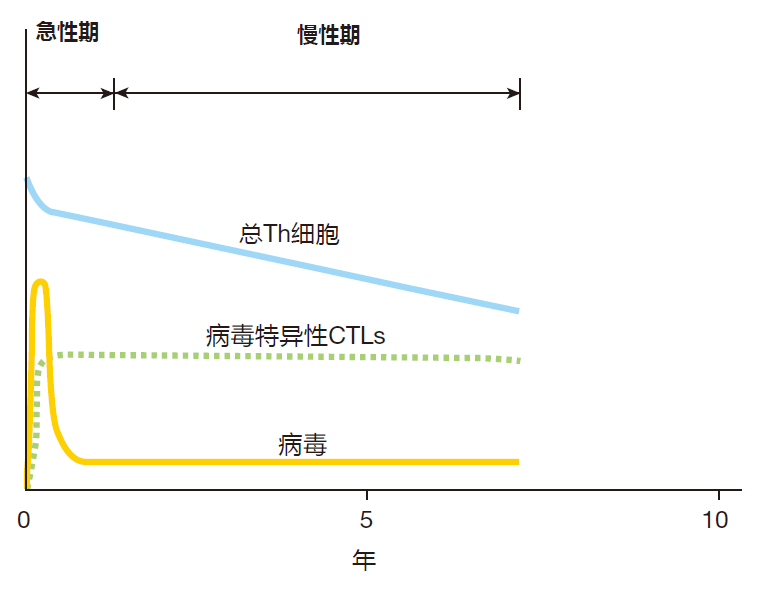
However, as the chronic phase progresses, the total number of helper T cells slowly decreases, because these cells are killed as a consequence of the viral infection. Eventually there are not enough Th cells left to provide the help needed by virus-specific CTLs. When this happens, the number of CTLs also begins to decline, and the viral load increases – because there are too few CTLs left to cope with newly infected cells.
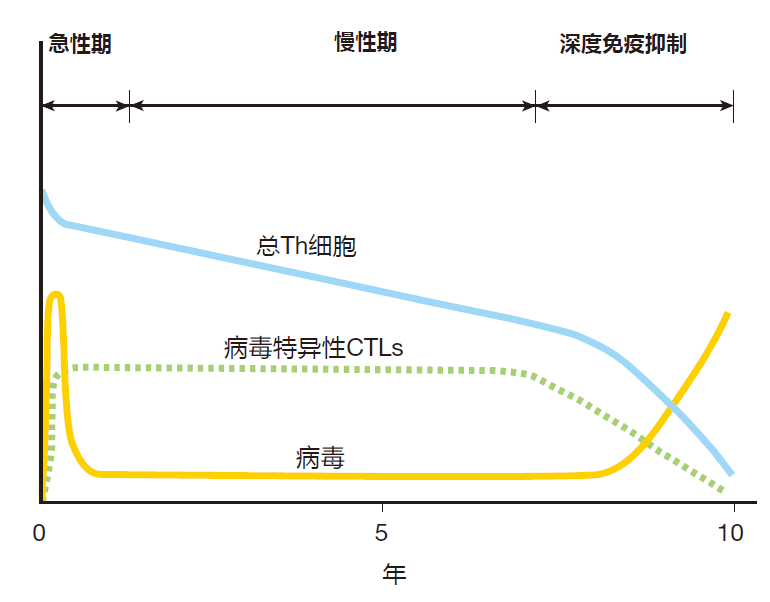
In the end, the immune defenses are overwhelmed, and the resulting profound state of immunosuppression leaves the patient open to unchecked infections by pathogens that normally would not be the slightest problem for a person with an intact immune system. Sadly, these opportunistic infections can be lethal to an AIDS patient whose immune system has been destroyed.
HIV-1 versus the immune system
Why is HIV-1 able to defeat an immune system that is so successful in protecting us from most other pathogens? There are two parts to this answer. The first has to do with the nature of the virus itself. All viruses are basically pieces of genetic information (either DNA or RNA) with a protective coat. For the AIDS virus, this genetic information is in the form of RNA which, after the virus enters its target cell, is copied by a viral enzyme (reverse transcriptase) to make a piece of "complementary" DNA (cDNA). Next, the DNA of the cell is cut by another enzyme carried by the virus, and the viral cDNA is inserted into the gap made in the cellular DNA. Now comes the nasty part.
Once the viral DNA has been inserted into a cell's DNA, it can just sit there, and while the virus is in this "latent" state, the infected cell cannot be detected by CTLs. Recent data suggests that it takes only five to ten days for HIV-1 to initiate a latent infection and establish a stealth reservoir of virus in these "sanctuary" cells. Sometime later, in response to signals that are not fully understood, the latent virus can be "reactivated," additional copies of the virus can be produced, and more cells can be infected by these newly minted viruses.
The fact that a reservoir of virus which is invisible to the immune system can be established within a week or so of infection is a serious problem. After all, a week into the infection, the adaptive immune system is still being activated. Consequently, the major responsibility for stopping the virus before it can get a "foothold" falls to the innate system. And the innate immune system usually is not up to the task.
So the ability to quickly establish a latent infection which cannot be detected by CTLs is one property of HIV-1 that makes this virus such a problem for the immune system. But it gets worse. The reverse transcriptase enzyme used to copy the HIV-1 RNA is very error-prone: It makes a "mistake" almost every time it copies a piece of viral RNA. This means that most of the new viruses produced by an infected cell are mutated versions of the virus which originally infected that cell.
And some of these mutations may enable the newly made viruses to evade the immune system. For example, the virus can mutate so that a viral peptide that formerly was targeted by a CTL no longer can be recognized, or no longer can be presented by the MHC molecule that the CTL was trained to focus on. In fact, it has been shown that it only takes about ten days for these escape mutants to arise. When such mutations occur, the original CTL will be useless against cells infected with the mutant virus, and new CTLs which recognize a different viral peptide will need to be activated. Meanwhile, the virus that has escaped from surveillance by the obsolete CTLs is replicating like crazy, and every time it infects a new cell, it mutates again. Consequently, the mutation rate of the AIDS virus is so high that it usually can stay one step ahead of CTLs or antibodies directed against it.
So two of the properties of HIV-1 that make it especially deadly are its ability to establish an undetectable, latent infection and its high mutation rate. But that's only half the story. The other part has to do with the cells HIV-1 infects. This virus specifically targets cells of the immune system: helper T cells, macrophages, and dendritic cells.
The "docking" protein that HIV-1 binds to when it infects a cell is CD4, the co-receptor protein found in large numbers on the surface of helper T cells. This protein also is expressed on macrophages and dendritic cells, although they have fewer CD4 molecules on their surface. By attacking these cells, the AIDS virus either disrupts their function, kills the cells, or makes them targets for killing by CTLs that recognize them as being virus-infected. So the very cells that are needed to activate CTLs and to provide them with help are damaged or destroyed by the virus.
Even more insidiously, HIV-1 can turn the immune system against itself by using processes which are essential for immune function to spread and maintain the viral infection. For example, HIV-1 can attach to the surface of dendritic cells and be transported by these cells from the tissues, where there are relatively few CD4 + cells, into the lymph nodes, where huge numbers of CD4 + T cells are located. Not only are helper T cells within easy reach in the lymph nodes, but many of these cells are proliferating, making them ideal candidates to be infected and become HIV-1 "factories."
Also, AIDS viruses that have been opsonized either by antibodies or by complement are retained in lymph nodes by follicular dendritic cells. This display is intended to help activate B cells. However, CD4 + T cells also pass through these forests of follicular dendritic cells, and as they do, they can be infected by the opsonized AIDS viruses. And because virus particles typically remain bound to follicular dendritic cells for months, lymph nodes actually become reservoirs of HIV-1. The net result is that HIV-1 takes advantage of the normal trafficking of immune system cells through lymph nodes, and turns these secondary lymphoid organs into its own playground.
In summary, the pathological consequences of an HIV-1 infection are the result of the virus' ability to slowly destroy the immune system of the patient, leading to a state of profound immunosuppression which makes the individual an inviting host for life-threatening infections. The virus is able to do this because it can rapidly establish a latent, "stealth" infection, because it has a high mutation rate, because it preferentially infects and disables the immune system cells that normally would defend against it, and because it uses the immune system itself to facilitate its spread throughout the body.
Living with AIDS
Untreated, most people infected with HIV-1 die within ten years. Fortunately, for those who can afford it, antiretroviral treatment (ART) is now available. World-wide, about 20 million people receive these drugs. This chemotherapy targets specific aspects of the viral replication cycle and can lengthen the life of an AIDS patient by many years. Nevertheless, ART does not eliminate the virus from the body of the patient – because it cannot eradicate the reservoir of latent virus "hiding" in infected CD4 + cells. In most cases, lifelong ART is required to control the disease, and this type of chemotherapy is not without side effects. Indeed, for those on ART, there is an increased risk for cancer and cognitive disorders, as well as kidney, liver, bone, and heart disease. As a result, the average life span of an AIDS patient on ART is about 20 years less than that of a person who has not been infected with HIV-1.
Interestingly, for a very small fraction of untreated individuals infected with HIV-1 (roughly 0.3%), their immune system is able to control the infection for a relatively long period of time. In fact, some of these elite controllers have almost undetectable levels of virus, and have remained symptom-free for as long as 30 years. As you might expect, immunologists are very interested in understanding how the immune system of an elite controller deals with a viral infection that is deadly for most other humans. Although the story is far from complete, there are some clues.
One consistent finding is that the innate and adaptive defenses of elite controllers seem to fire up more quickly after the initial infection than does the immune system of "ordinary" humans. Several possible reasons for this quick response have been discovered. For example, the pattern-recognition receptors of some elite controllers trigger unusually vigorous secretion of IFN-α and IFN-β by cells of the innate immune system. IFN-α and IFN-β activate genes within AIDS-infected cells that encode proteins which limit the efficiency of replication of the virus.
In addition, these "warning cytokines" can cause infected cells to die by apoptosis, destroying the viruses that are replicating inside them.
In Lecture 4, I noted that one reason MHC molecules are so polymorphic is to increase the probability that at least some individuals in the population will have MHC molecules that can bind to and present an invader's peptides.
This idea is supported by the finding that certain class I MHC molecules are found much more often in elite controllers than in the general population. The thinking here is that because these MHC molecules efficiently present HIV-1 peptides, killer T cells will be activated earlier in an infection when the number of infected cells is still small.
In addition, when CTLs from elite controllers are tested in the laboratory, they tend to be more vicious killers than CTLs from patients who cannot control the infection. This seems to be due to the ability of these "super CTLs" to mobilize the killing enzyme granzyme B, and deliver it into its target cells. Again, the thinking is that these CTLs kill faster, and can control the infection before it gets out of hand.
Of course, the hope is that if the unique features of the immune system of elite controllers can be understood in more detail, this information may be helpful in devising new treatments for persons infected with HIV-1. It is important to understand, however, that elite controllers are still infected: Their immune system has not defeated the virus. It has just controlled the viral infection for an extended period of time, and these individuals continue to have reservoirs of latently infected CD4+ T cells.
So far, there is only one documented case of an AIDS patient who appears to have been treated and cured of the disease: the so-called "Berlin patient," Timothy Ray Brown. After he was infected with HIV-1, Mr. Brown developed acute myelogenous leukemia. When he was treated for that disease, he twice had his immune system destroyed by chemicals or radiation and reconstituted with stem cell transplants. The stem cell donor in both procedures had deletions in his genes for CCR5, the most common co-receptor for the AIDS virus. As of this writing, the Berlin patient is still free of cancer and HIV-1.
REVIEW
Mutations that are inherited or that arise spontaneously can cause the immune system to function suboptimally.
Other immunodeficiencies arise when the immune system is suppressed by drugs or disease. Today, millions of humans are immunodeficient as a result of infection with the AIDS virus. Untreated, most AIDS patients succumb to infections which the immune system of a healthy individual could easily defeat. HIV-1 goes at the immune system "head on" by infecting and destroying the very immune warriors which might otherwise defend against the attack.
The virus uses the immune system to facilitate its spread throughout the body, and it can establish a "hidden" reservoir of virus within the immune system cells of an infected individual. In addition, because the virus mutates rapidly, killer T cells, which can recognize and kill infected cells, quickly become "obsolete," allowing the virus to stay one step ahead of the immune defenses.
Anti-retroviral treatment, a form of chemotherapy, can be used to extend the life of patients infected with HIV-1.
However, these treatments are expensive and can have serious side effects. Some "elite controllers" are chronically infected with the virus, but remain asymptomatic for long periods of time. Immunologists are eagerly examining the immune system of these "lucky few" to try to determine why they are able to control an infection which is lethal to so many others.


