8.8 CHANGES IN REPRODUCTIVE SYSTEM
Human reproductive aging has, historically, been associated with female aging, and it reflects three important changes. First, for women, fertility comes to a rather abrupt end with the cessation of menses and the onset of menopause, usually at 50–60 years of age. Second, ovarian production of sex-linked hormones, such as estrogen and progesterone, drops dramatically at the time of menopause. And third, a time-dependent decline in the genetic quality of eggs leads to an increased occurrence of genetically damaged embryos, birth defects, and spontaneous abortions. As you learned in previous chapters and learn more about in Chapter 9, the end of a woman's reproductive life span also correlates with other time-dependent physiological dysfunctions.
Men do not experience an abrupt end to fertility. This observation has often been misinterpreted to mean that men do not experience a decline in their ability to conceive. In the past couple of decades, researchers have found that men undergo many of the same time-dependent reproductive changes that are observed in women. Although men do not experience an end to their reproductive life span, male fertility and sex hormone production do decrease with age. Moreover, the genetic quality of sperm appears to decline with advanced age, resulting in an increased risk for genetic problems in offspring. In this section, we briefly examine how declining hormone production affects the reproductive systems of women and men.
Menopause is caused by declining secretion of sex hormones by gonads
The female reproductive system produces a single egg on a 28-day schedule called the menstrual cycle (Figure 8.44). The cycle begins when luteinizing hormone (LH) and follicle-stimulating hormone (FSH), secreted by the pituitary gland, stimulate an ovarian follicle—the structure containing a maturing ovum—to release its mature ovum and to secrete estrogen and progesterone into the blood. Estrogen and progesterone serve two functions: (1) they are necessary for the maintenance of female sexual characteristics and sex organs, and (2) they have a feedback effect on the ovary, stimulating development of the ovum in the follicle. Increasing blood concentrations of LH, FSH, and estrogen induce a developing follicle to rupture and release the ovum, a process known as ovulation. The ruptured follicle becomes a structure called the corpus luteum, which increases the secretion of progesterone. Increased blood concentrations of estrogen and progesterone also have a feedback effect on the pituitary gland, inhibiting the secretion of LH and FSH and ensuring that only one follicle develops a mature egg. Estrogen and progesterone prepare the uterus for possible implantation of a fertilized egg by stimulating cell growth in and blood flow to the endometrial wall. If fertilization does not occur, estrogen and progesterone levels fall, and the thickened layer of the endometrium is sloughed off in the menstrual flow. The fall in estrogen and progesterone also causes the pituitary to begin secreting LH and FSH, starting the cycle again.
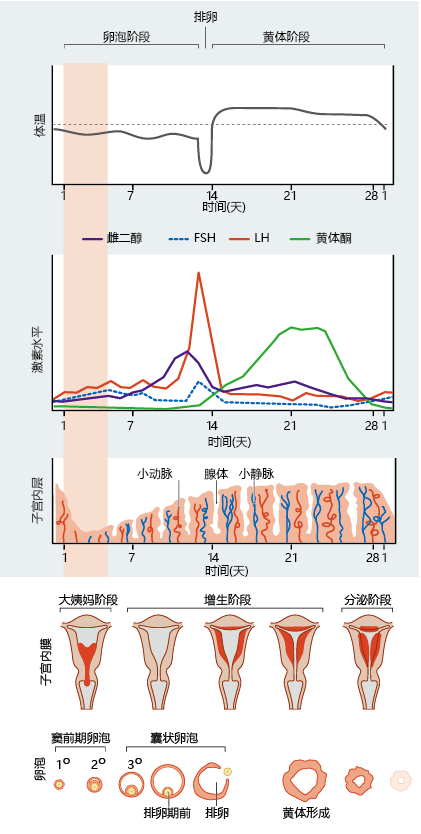
Figure 8.44 The menstrual cycle. These graphs and diagrams show the relationships among body temperature, hormone levels, uterine changes, and ovarian morphology over the course of the menstrual cycle. Antral follicles (also called Graafian follicles) are follicles at a late stage of follicle maturation.
At birth, the ovary contains a female's complete supply of oocytes, undeveloped eggs—about 750,000. The number of oocytes declines with age so that by the time a woman reaches menopause, at 50–60 years of age, fewer than 5,000 oocytes remain. The number of ovarian follicles falls to zero at the time of menopause. These two time-dependent ovarian changes ultimately lead to a cessation of estrogen and progesterone secretion by the gonads. It is the termination of the ovaries' production of these two hormones that marks the start of menopause.
The cessation of estrogen and progesterone production at menopause causes significant time-dependent changes, in addition to the loss of oocyte development. Without the monthly cycle of ovarian hormones, the uterus begins to shrink and can decrease in size as much as 70% within 15–20 years after menopause. The vagina becomes smaller and less elastic, and its endothelial wall thins. With the thinning (not to imply total loss) loss of endothelium, the vagina becomes less effective as a barrier against abrasion, resulting in an increase in pain and risk of injury during intercourse. The reduction in endothelial tissue also results in a reduced amount of glycogen secreted into the vagina. The pH of the vagina rises, resulting in an increased risk of infections.
Estrogen also has effects on many other, nonsexual organs and tissues. As discussed in Chapter 9, normal bone growth in women can be influenced by estrogen. The loss of estrogen during menopause results in significant bone mineral loss. The hot flashes experienced by many menopausal and postmenopausal women appear to be related to loss of estrogen's inhibition of LH secretion. Numerous studies have shown that the hypothalamic neurons that regulate the pituitary secretion of LH may also be involved in thermoregulation. Without estrogen, these neurons are stimulated, and this may affect thermoregulation. Finally, the loss of ovarian production of estrogen and progesterone has been related to an increasing risk for heart disease and cancer. Although the mechanism for this increased risk has yet to be elucidated, it is thought that estrogen acts as a “protective” agent against cell damage during the childbearing years.
Male fertility declines slightly with age
The issue of time-dependent alterations in men's ability to conceive at advanced ages has only recently become a topic of intensive research. It is now generally accepted that male fertility declines slightly with age. Anatomical changes cannot, in general, account for these declines.
Impotence, or erectile dysfunction, is the inability to achieve or maintain an erection and most often reflects secondary symptoms arising from other medical problems. Time-dependent alterations in blood flow to the penis have not been observed, although as men age, the erectile tissue of the penis becomes infiltrated with fibrous tissue. Although the penis can still become erect, the fibrous tissue results in a less stiff erection. The testes decrease slightly in both size and weight, but the extent of decrease is highly variable. Most of the time-dependent size changes in the testes can be accounted for by loss of cells in the seminiferous tubules, the structures responsible for sperm and testosterone production. Time-dependent anatomical changes in the epididymis, vas deferens (ductus deferens), urethra, and seminal vesicles that lead to changes in sexual function have not been widely observed.
The slight time-dependent decline in fertility in men most likely reflects changes in the cells of the seminiferous tubules. As a man ages, the sperm-supporting cells, called Sertoli cells, are slowly replaced by fibrous tissue, decreasing the total number of sperm per ejaculation. The number of sperm produced per Sertoli cell also appears to decline, although wide variation in this variable has been reported. The number of Leydig cells, the cells that produce testosterone, also declines. Testosterone is required for the initiation of sperm production. It is also required for the maintenance of healthy gametes. Reduced testosterone production has been suggested as one mechanism for the higher rate of genetic errors observed in sperm from older men.
Although a decline in serum testosterone concentration has been shown to correlate with a decrease in Leydig cell number, some studies suggest that alterations in the hypothalamic-pituitary-testicular axis may also be responsible for the reduced testosterone. Testosterone production in young men follows a circadian rhythm, a rhythm based on 24 hours. Production is highest between the hours of 4 and 8 a.m. and lowest around midnight. The circadian rhythm for testosterone corresponds to a similar rhythm for LH, the pituitary hormone primarily responsible for initiating testosterone production. Older men have both lower concentrations of testosterone and a loss of circadian rhythm in its production. Nonetheless, LH levels do not decline in proportion to the decline in testosterone, suggesting that, with aging, the Leydig cells become less responsive to the stimulatory effects of LH. Because a similar response is observed in women during menopause, the decrease in responsiveness of Leydig cells to LH has been named andropause.
Old age is not barrier to sexual activity
The time-dependent changes in reproductive organs in men and women have only a minor impact on sexual performance. In men, the erectile tissue of the penis accumulates fibrous material that can alter the speed of erection and the stiffness of the tissue. Impotence, the inability to engage in sexual intercourse because the penis is not sufficiently erect, is not a normal time-dependent condition. It occurs in only about 15%–20% of men over the age of 60. Although normal time-dependent alterations in the testes, seminal vesicles, and prostate do affect fertility and increase the risk of disease, the changes in these organs do not affect sexual performance in the majority of men.
Some men do experience sexual dysfunction as a result of prostate cancer. There exists considerable controversy as to whether it is the cancer or the specific cancer treatment that influences sexual dysfunction associated with prostate cancer. Regardless, given that the prevalence rate of prostate cancer stands at 165 men/100,000 (0.165%), the number of men experiencing sexual dysfunction would be very low. Benign prostatic hyperplasia (BPH), noncancer enlargement of the prostate, has been suggested to result in sexual dysfunction in men. However, reliable data on this subject do not exist. Some current data suggest strongly the BPH is more likely the result of obesity and other unhealthy lifestyle choices than aging, per se.
In women, the thickness of the vagina wall decreases with age, resulting in changes that can affect sexual performance and health. The lubricating secretion of the mucosal cells declines, increasing friction during sexual intercourse and the possibility of pain and minor physical trauma. However, recent evidence suggests that women who participate in regular sexual activity show little decline in mucosal cell secretions.


