8.7 CHANGES IN IMMUNE SYSTEM
Roy Walford(1924–2004), who conducted groundbreaking research in biogerontology, proposed 40 years ago that declining immune function might cause normal aging. This proposal, known as the immunological theory of aging, has yet to be completely tested, but we do know that mortality rate due to infectious diseases can be three to four times greater in people over the age of 65 years than in younger age groups. Moreover, the percentage of people over 65 who are protected by vaccination against novel influenza viruses is only about half that found in the younger population. Clearly, advanced age compromises the effectiveness of the human immune system.
In this section, we explore the physiology of the immune system and discuss how aging affects the body's ability to repel attacks from potentially harmful invaders. Human immunity is an extremely complex function involving several redundant systems that work together to prevent infection. This complexity precludes a thorough discussion of the human immune system in this text. Instead, we focus on some functions that have been shown to decline with age.
Innate immunity provides first line of defense against infection
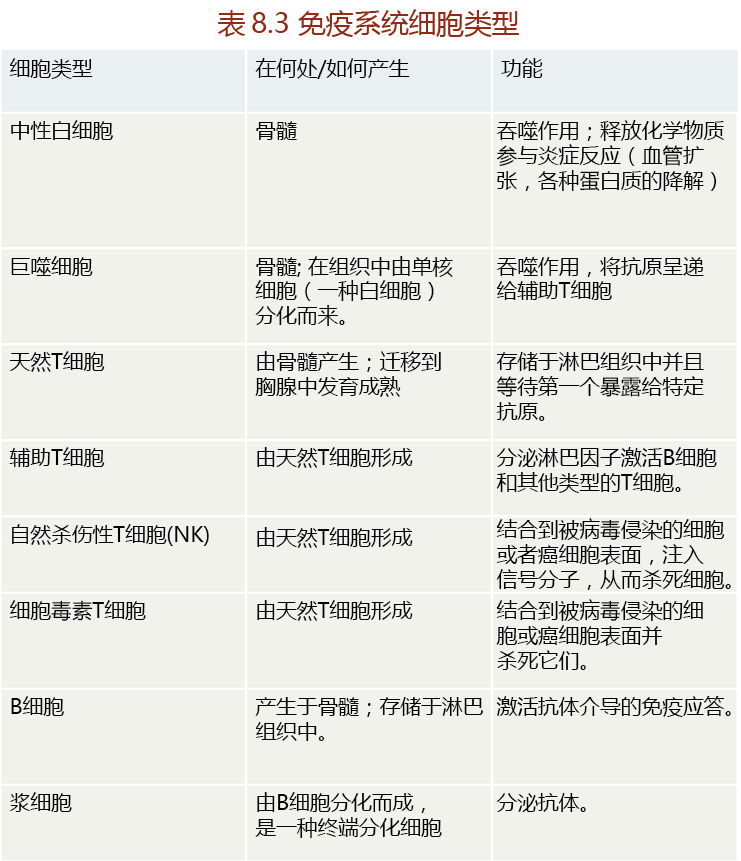
Humans have two separate but equally important immune systems: innate immunity and acquired (adaptive) immunity. We are born with a fully intact innate, or natural, system of immunity that either prevents infection or reacts quickly to nonself agents. Innate immunity prevents infection by erecting barriers such as the skin, the mucosal linings of body cavities, and digestive acids and proteases. If a foreign substance gets through these protective barriers, the innate system stimulates phagocytosis, the process of engulfing and ingesting foreign particles; this is carried out by specialized blood- and tissue-borne cells such as neutrophils and macrophages (TABLE 8.4).
Neutrophils are white blood cells that circulate in the blood and migrate into tissue where foreign matter has entered the body. Both neutrophils and macrophages can carry out phagocytosis, but tissue macrophages are much larger than neutrophils and phagocytose larger particles. Small fragments of the macrophage-digested invader are carried to the lymph tissue by specialized cells called dendritic cells, which activate the acquired immune system.
When tissue injury occurs, the affected site releases compounds, such as histamines and prostaglandins that increase blood flow, capillary permeability, and migration of neutrophils to the area. Collectively, these responses characterize inflammation and activate the phago-cytotic process of the innate immune system (Figure 8.42). If bacteria or other foreign agents enter a wound, tissue macrophages surround the invaders within minutes and begin to phagocytose the organisms. Macrophages also induce the secretion of cytokines and chemokines, proteins that attract white blood cells, such as neutrophils, to the wound by chemotaxis. Because the tissue concentration of macrophages is low, neutrophils perform most of the phagocytosis at the inflammation site over the first few hours after injury.
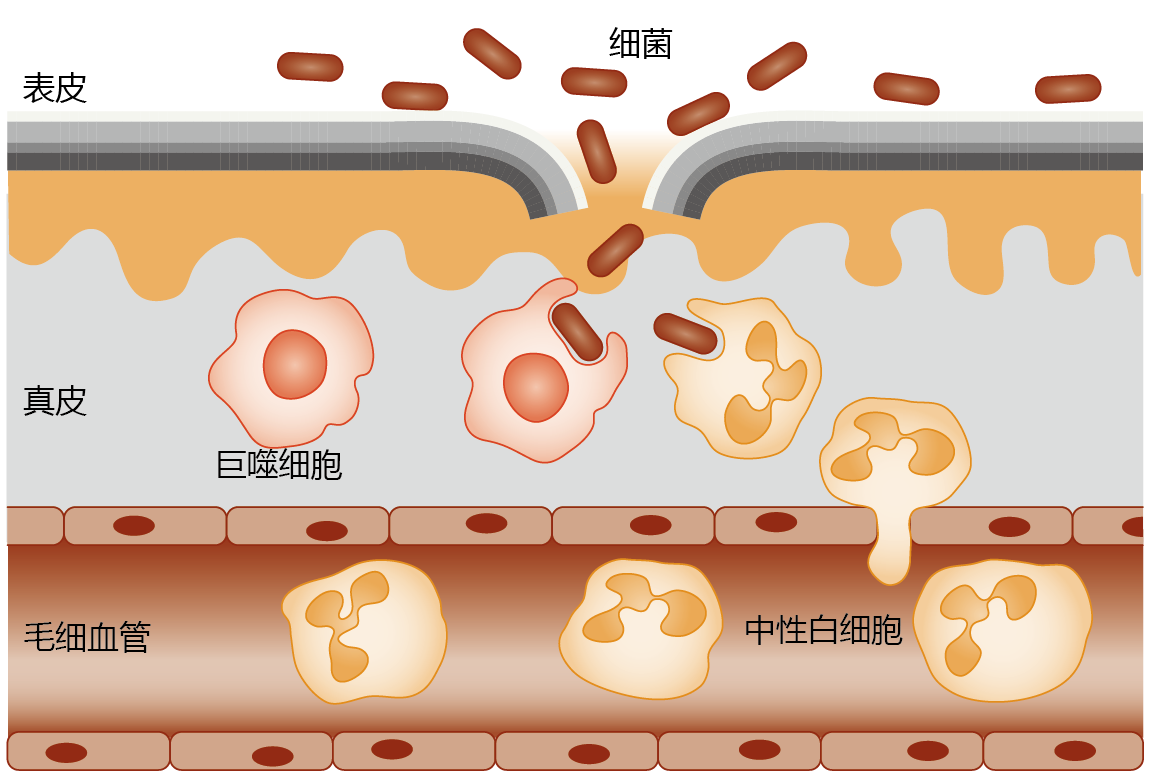
Figure 8.42 Inflammation and innate immunity. If bacteria breach the protective barrier of the innate immune system (represented here by a cut in the skin epidermis), the area around the invasion site becomes inflamed. The tissue macrophages are the first line of defense against the invading bacteria and begin the phagocytotic process within minutes. Inflammation also increases capillary permeability, allowing passage of neutrophils into the tissue. Infiltration by neutrophils, occurring by chemotaxis, and their subsequent phagocytotic activity start within an hour of injury and may last for 2–3 days.
Acquired immunity relies on lymphocytes reacting to antigens
The same processes that maintain life—breathing and eating—also expose human beings to millions of harmful bacteria, viruses, and toxins. While innate immunity provides an effective barrier against invasion by these agents, the sheer number of harmful agents in the environment can easily overwhelm this system, allowing many invaders to get through the innate immune system defenses. Once this happens, the responsibility for protecting the body from infection falls on the lymphocytes—T cells and B cells—of the acquired immune system (see Table 8.4).
T cells are small white blood cells (lymphocytes) that are made in the bone marrow and mature in the thymus gland before being stored in the lymph tissue. At this stage, they are naive T cells, not yet transformed into immune cells that attach to and kill invaders. T cells are activated when dendritic cells, carrying fragments of macrophage-digested foreign particles, enter the lymph tissue. The binding of these fragments to the surface receptors of a specific type of T cell stimulates the production and release of three different T-cell clones from the lymph tissue. These T-cell clones are responsible for destroying the invading antigen, any substance capable of inducing a specific immune response. The vast majority (75%–80%) of activated T-cell clones released from the lymph tissue are helper T cells. As the name implies, helper T cells “help” the immune system respond adequately to the invader. Helper T cells do not attack the antigens directly; rather, they secrete compounds known as lymphokines (interleukins and interferon) that stimulate the proliferation and differentiation of other cells that do attack the antigen (Figure 8.30). For example, lymphokines secreted by helper T cells induce B cells to synthesize and release antibodies specific to the antigen. The other two important types of T cells are cytotoxic T cells and natural killer (NK) cells produced for specific purposes.
In brief, antigens entering lymph tissue are destroyed by macrophages, which then present antigen fragments to naive T cells (Figure 8.43). Binding of antigen fragments to the surface of the naive T cells induces differentiation and proliferation of helper T cells and NK cells or cytotoxic T cells. Helper T cells secrete lymphokines that stimulate the production of antibodies in activated B cells. NK cells attach to antigens on the membranes of virus-infected cells and inject toxic proteins that destroy the cells. Naive B cells engulf macrophage-produced antigen fragments, and this induces the B cells to differentiate into plasma cells that are capable of synthesizing antibodies. The antibodies form extremely strong bonds with the antigen causing a change in the antigen configuration. As a result, the antigen can no longer bind to body cells and cause damage. The antibodies also mark the antigen for degradation by other immune cells.
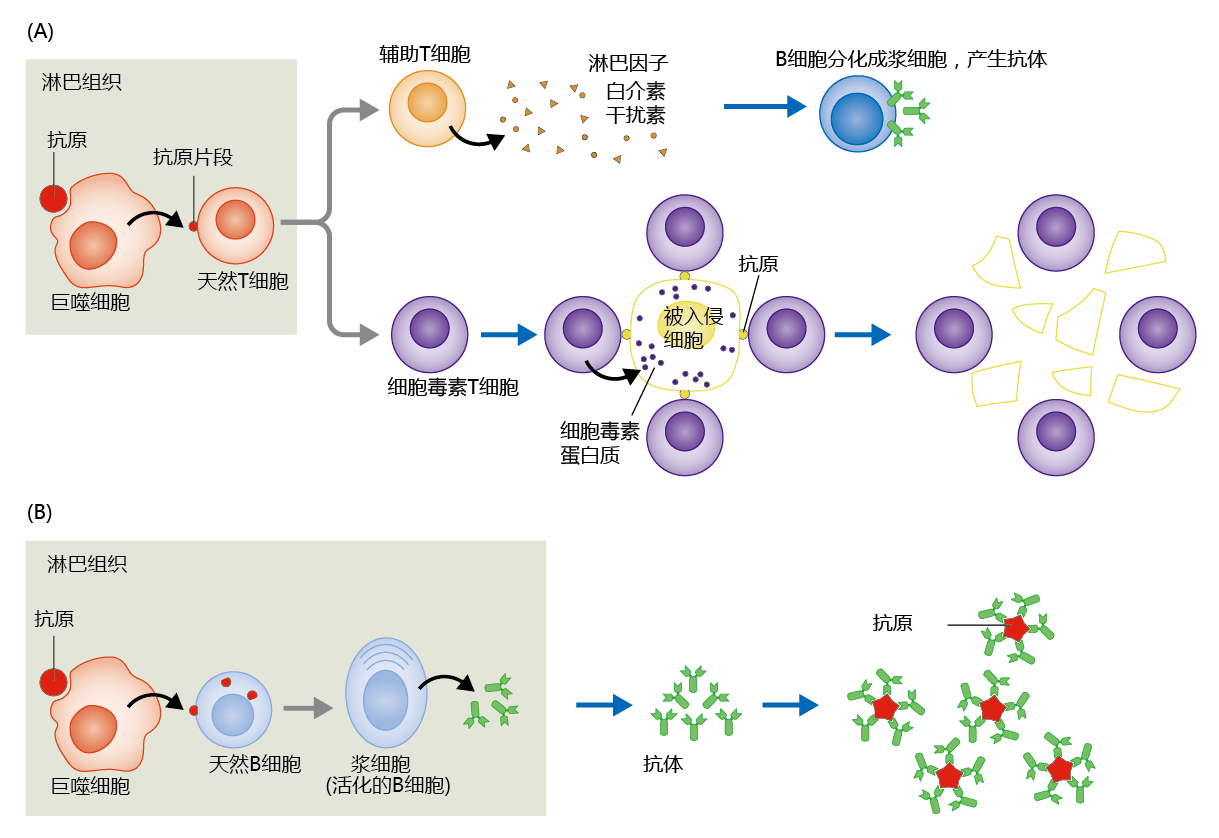
Figure 8.43 Mechanism of T-cell and B-cell immunity. (A) Macrophages from the affected tissue present partially digested antigen fragments to naive T cells. Naive T cells are transformed into helper T cells, cytotoxic T cells (as shown here), or NK cells. (B) Helper T cells activate B cells, transforming them into plasma cells—immune cells that secrete antibodies. The antigen is immobilized by the strong bonding of antibodies.
The first time an antigen is encountered, it takes several days for the body to produce sufficient T cells and B cells to neutralize it. After the antigen has been eliminated and no longer poses a threat to the body, the majority of T and B cells are destroyed. But some T and B cells remain after this first encounter with an antigen; these are called memory cells. Memory cells contain surface receptors that are specific to the antigen that was eliminated, and they are able to produce clones quickly when encountering that antigen again. In fact, enough clones can be produced in a single day to effectively eliminate the antigen at the next exposure.
Phagocytotic function of neutrophils and macrophages declines with age
The effectiveness of the innate immune system relies, in large part, on the ability of neutrophils and macrophages to infiltrate the affected area. The number of neutrophils and macrophages available to the innate immune system does not decline with age, and the synthesis of these cells from hematopoietic stem cells in the bone marrow appears to be well conserved throughout life. Therefore, time-dependent functional loss in the innate immune system probably reflects a loss of the phagocytotic function of the neutrophils and macrophages (TABLE 8.5).
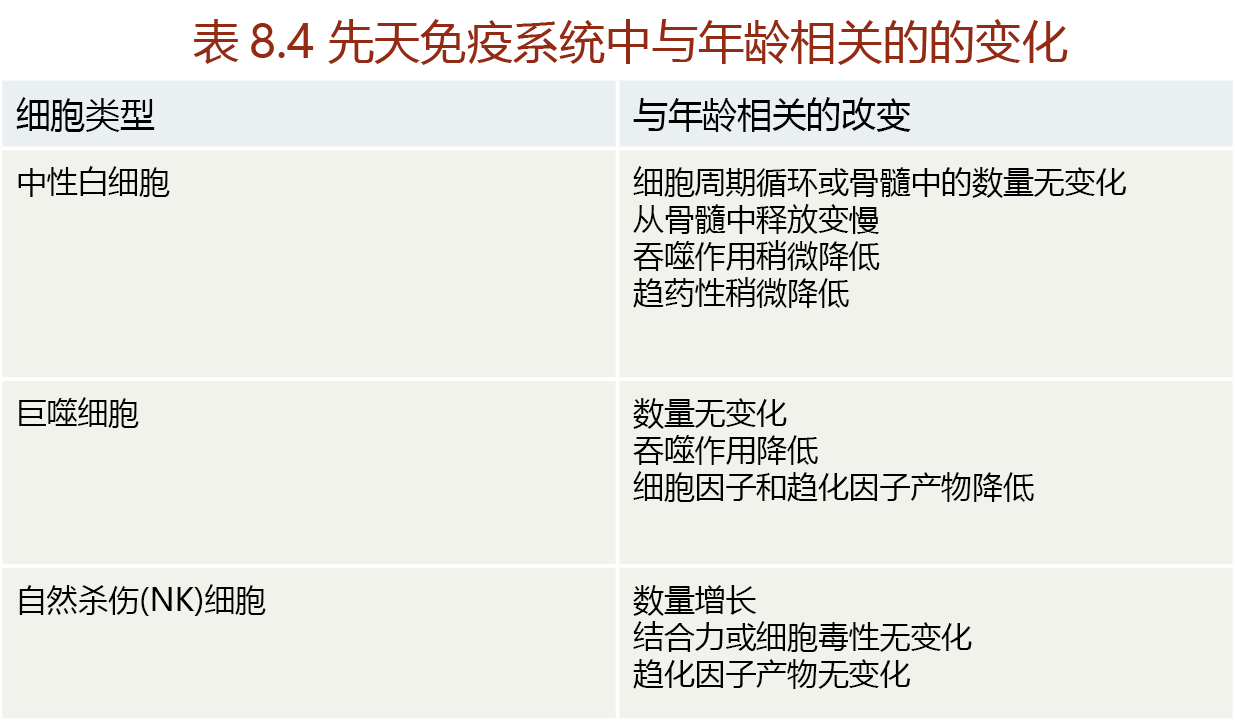
The decline in phagocytotic function of the innate immune system may explain why wound healing takes significantly longer in postreproductive individuals than in younger populations. The healing delay may also result from a decline in cytokine and chemokine production by macrophages. Recall that macrophages stimulate the release of neutrophils from bone marrow stores through cytokine production and direct the neutrophils to the injured site through chemokine release. Attenuation of cytokine and chemokine release would reduce the number of neutrophils arriving, or delay arrival, at the wound site. In addition, two other time-dependent changes contribute to a delay in wound healing. First, the density of vessels and capillaries in the skin decreases with age. Because the healing of skin wounds relies on the ability of neutrophils to diffuse from capillaries into the damaged tissue, a lower blood vessel and capillary density would decrease the total number of neutrophils arriving at the wound site. Second, repair of skin wounds relies on the ability of fibroblasts to secrete the necessary connective tissues that bind replacement cells together. As we saw earlier in the chapter, the mitotic activity of aged skin cells declines, so there are fewer fibroblasts to provide the proteins for new connective tissue. Together, the decline in vessel density and in fibroblast production results in an increase in healing time and an increased risk of infection.
Production of naive T cells, number of B cells, and effectiveness of antibodies all decline with age
Recall that the thymus gland, a small gland just behind the breastbone, is the site where T cells develop. Aging results in a significant atrophy of the thymus gland, which causes a time-dependent reduction in the production of naive T cells. Thymic involution reflects the shrinkage of the epithelial space—the location of T-cell development—and replacement of the population of maturing T cells with connective tissue. It has been estimated that the epithelial space in the thymus declines by 90% by the age of 70. Interestingly, the epithelial cells that remain within the involuted thymus do not seem to have attenuated T-cell production. That is, the time-dependent decline in T-cell production reflects solely the loss of thymic epithelial cells. With the decline in production of naive T cells, the ratio of memory T cells to naive T cells increases. Moreover, the capacity for the clonal expansion of memory T cells into helper T cells, a vital process in mounting an appropriate immune response to an antigen, may also decline with age.
The time-dependent decrease in T-cell production might also reflect altered function of hematopoietic stem cells (HSCs), bone marrow stem cells responsible for renewing blood cells. HSCs give rise to cells of the innate and adaptive immune system, also known as myeloid and lymphoid cells, respectively. Although a time-dependent decrease in absolute number of HSCs has yet to be established, research in mice has consistently shown that the production of myeloid cells outpaces that of lymphoid cells as the animal ages. This would suggest that the number of T cells decreases with age, that is, reduced adaptive immunity. Caution should be used, however, when making the conclusion that reduced adaptive immunity results from a decrease in HSC lymphoid cells. Recall that immature T cells are made in the bone marrow and mature in the thymus gland (see previous discussion). Thus, the time-dependent decrease in lymphoid cells could reflect a feedback mechanism in which the involution of the thymus gland “tells” the HSCs to reduce differentiation into lymphoid cells.
Together, the time-dependent involution of the thymus gland, decline in peripheral naive T cells, and decreased clonal expansion of memory T cells into helper T cells explain the overall time-dependent decline in immune function. Recall that the acquired immune system uses memory T cells to shorten the time required to respond to an invader. Helper T cells also stimulate the release of antibodies by B cells. If the memory T cell does not function at peak performance, the immune response is blunted, and the person is at greater risk for developing complications as a result of the invading antigen. The time-dependent decline in naive T cells reduces the individual's ability to respond to a novel antigen. This may help explain why, as noted previously for influenza, vaccinations are less effective in the elderly than in the younger population.
The number of mature B cells also falls with age, reflecting decreased B-cell synthesis in the bone marrow; this leads to an increase in the ratio of memory B cells to mature B cells, although the total peripheral B-cell component does not decline with age. As occurs for memory T cells, memory B cells have a lower capacity for clonal expansion in older people. With the time-dependent decline in peripheral mature B cells, the ability to mount an immune response to novel pathogens is blunted.
Antibody function also declines with age, reflecting the diminution in number and function of helper T cells. That is, the decreased clonal expansion of memory T cells into helper T cells reduces the amount of cytokine release, which, in turn, reduces the release of antibodies from B cells. In addition, the number of high-affinity antibodies (antibodies with multiple antigen-binding sites) also falls with age. This results in a weakening of the immune response, because the strength of the antigen-antibody bond determines, for the most part, antibodies' effectiveness in neutralizing invaders.


