9.1 NERVOUS SYSTEM AND NEURAL SIGNALS
The nervous system is the most complex system in the human body. Every movement, every breath, every heartbeat, every sensory perception begins in the nervous system. Our nervous system has three overlapping functions: sensory input, integration, and motor output. These functions are performed by the brain, the spinal cord, the sensory receptors that receive stimuli, the effector cells that carry out the body's responses to stimuli, and a network of nerves that carry information to various parts of the body. The brain and spinal cord make up the central nervous system (CNS), and the nerves outside of the CNS make up the peripheral nervous system (PNS) (Figure 9.1). All nerves communicate their messages between the CNS and the rest of the body through a combination of electrical and chemical signals, generated by the exchange of ions across the nerve cell (plasma) membrane. In this section, we present an overview of the human nervous system and examine the nature of neural signals in the normally functioning system.
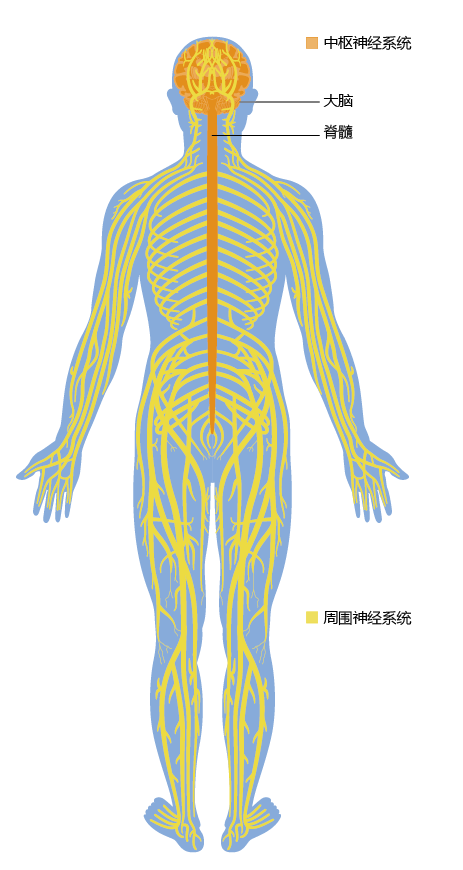
Figure 9.1 Gross anatomy of the human nervous system. The CNS consists of the brain and the spinal cord. Nerves originating from the CNS make up the PNS.
Nervous system is composed of neurons and supporting cells
Neurons, or nerve cells, are the cells that conduct messages along the communication pathways of the nervous system. There are three main types of neurons: sensory neurons, which convey sensory input about the external and internal environments to the CNS; motor neurons, which convey motor output from the CNS to effector cells (muscle or gland cells); and interneurons, which integrate sensory input and motor output. Although neurons vary somewhat in structure depending on their function, most consist of three general components: a cell body, dendrites, and an axon (Figure 9.2).
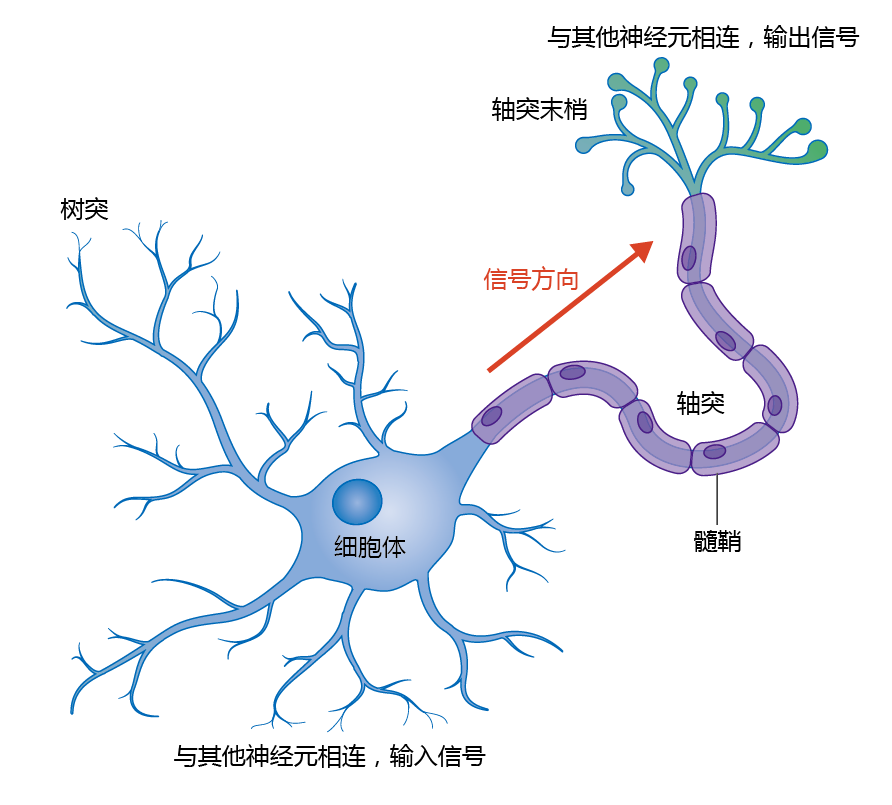
Figure 9.2 Anatomy of a neuron.
The cell body contains the nucleus and a variety of other cellular organelles, which carry out the normal functions of the cell. The dendrites are branch-like structures that extend from the cell body, increasing the surface area of the neuron. The dendrites receive signals from other neurons and sensory receptors and convey them to the rest of the neuron. The axons are long tubular extensions that carry signals from the cell body to their tips, the axon terminals. The axon terminals connect with the dendrites of other neurons. Many neurons have a single axon, which varies in length. For example, the axons that control fine movements in the fingers originate in cell bodies within the spinal cord and extend all the way to the hand, whereas other axons may be less than 1 mm in length. In addition, the axon may be branched, with each branch giving rise to specialized endings called synaptic terminals. The site of contact between a synaptic terminal and another cell is called a synapse. As discussed, the synapse is the location where one neuron communicates with another.
Axons are often surrounded by an insulating layer called the myelin sheath. In the PNS, the myelin sheath is formed from supporting cells called Schwann cells. In the CNS, the myelin sheath is produced by supporting cells called oligodendrocytes.
Membrane potentials establish conditions for neural signal transmission
All nerves generate impulses, or electrical signals, that transmit information to different parts of the body. These impulses depend on the flow of ions across the plasma membrane of neurons. All living cells have a difference in electrical charge across their plasma membranes. The membrane potential is the difference in voltage, or electrical potential, between the interior and exterior of the cell. The membrane potential exists because of differences in ion concentrations in the intracellular and extracellular fluids. The extracellular fluid contains a greater concentration of sodium (Na+) and chloride (Cl−) ions, while the intracellular fluid (cytosol) contains a greater concentration of potassium (K+) ions.
Although all cells have a membrane potential, only neurons and muscle cells are excitable, capable of changing the electrical state of their membranes. The size of the membrane potential determines a membrane's readiness or ability to propagate an electrical signal—the greater the membrane's potential, the greater the probability that it can undergo an electrical event. For a neuron in its resting (unexcited) state, the resting membrane potential is typically −70 millivolts (mV). By convention, the voltage outside the cell is zero, so a negative membrane potential indicates that there are more negative charges on the inside of the cell than on the outside (Figure 9.3). A change in potential that causes the membrane to become less polarized (less negative on the inside) compared with the resting membrane potential is called depolarization. A change that causes an increase in the potential is called hyperpolarization.

Figure 9.3 Membrane potentials and threshold potentials. The resting membrane potential of the neuron is –70 mV, making it highly polarized and ready for changes in electrical activity. When a triggering event occurs, the membrane potential begins to change slowly in the positive direction (less negative inside the cell). At approximately –55 mV, the threshold potential is reached, and Na + channels open, causing a rapid depolarization. At +30 mV, depolarization stops and begins to reverse (repolarization), returning the neuron to its resting membrane potential.
Recall from Chapters 4 and 8 that the plasma membrane is a phospholipid bilayer. Because ions are electrically charged, they cannot directly diffuse across the lipid of the membrane. The plasma membranes of neurons contain voltage-gated ion channels, which allow the movement of Na + and K + ions into and out of the cell. These channels have gates that open and close in response to electrical signals. This allows the cell to change its membrane potential in response to stimuli, or triggers, that the cell receives (Figure 9.4). When a small section of the cell membrane receives a triggering event, such as a thought by your brain to move your index finger, the event initiates an action potential. The triggering event causes the membrane to become more permeable to Na+, which begins to pass through the membrane into the intracellular compartment, starting depolarization. The reversal of charge occurs slowly until it reaches a threshold potential of about −55 mV. The threshold potential causes gated Na+ channels to open, resulting in an explosive depolarization and the creation of an action potential. When the action potential reaches about +30 mV, K+ channels open, and the flow of K+ out of the cell begins the repolarization process. The ending of the action potential in one segment of the membrane is the triggering event for initiation of the next action potential.
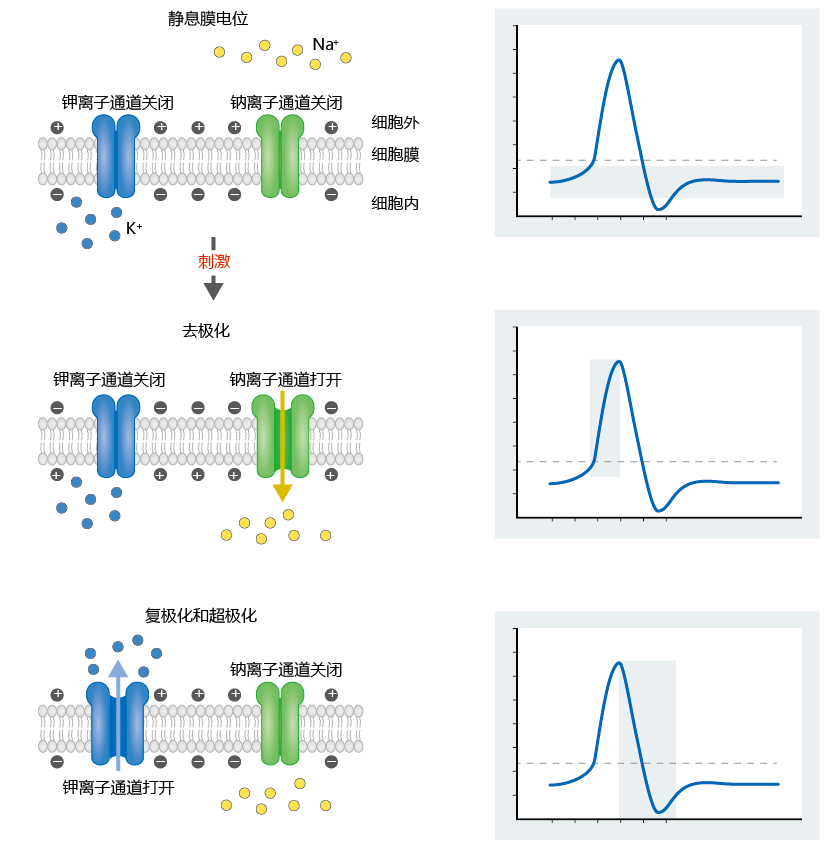
Figure 9.4 The role of voltage-gated ion channels in changes in membrane potential. A triggering event causes a slow depolarization as the membrane becomes more permeable to Na+. Sodium channels open when the membrane potential reaches –55 mV. The depolarization continues until the action potential reaches about +30 mV. Then the Na+ channels close and the K+ channels open. Potassium exits the cell, causing reversal of the depolarization process (repolarization). When the membrane potential hits –80 mV, the K+ channels close, and the membrane returns to its resting potential.
Neurotransmitters chemically link neurons together at synapse
Axon terminals do not come into physical contact with dendrites. Rather, the axon terminal of one neuron forms a chemical junction with the dendrites of another neuron (Figure 9.5). The transmitting cell is called the presynaptic neuron, and the receiving cell is called the postsynaptic neuron. The space between the two neurons is called the synaptic cleft. Together, the presynaptic neuron, postsynaptic neuron, and synaptic cleft make up the synapse. The ends of the presynaptic cell, called synaptic knobs, contain synaptic vesicles. Each vesicle contains thousands of neurotransmitter molecules, chemicals that are released into the synaptic cleft in response to an action potential.
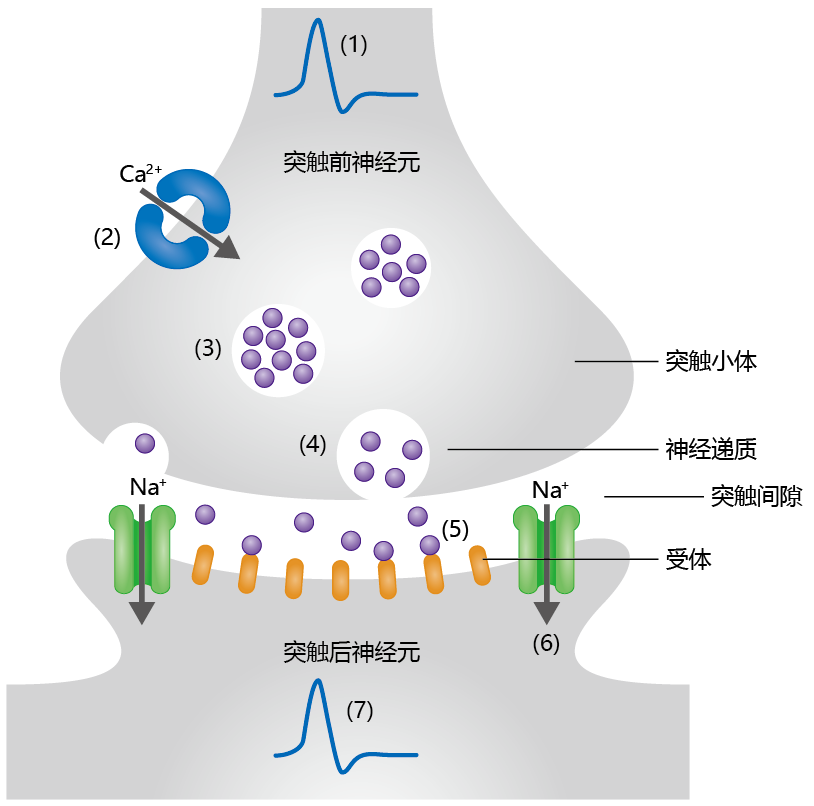
Figure 9.5 General mechanism of signal propagation between two neurons. (1) Generation of an action potential in the presynaptic cell causes (2) Ca2+ channels to open. The influx of Ca2+ into the synaptic knob causes (3) synaptic vesicles to move to the membrane and (4) undergo exocytosis, releasing neurotransmitter molecules into the synaptic cleft. (5) Binding of neurotransmitter molecules to receptors on the postsynaptic neuron induces (6) the opening of Na+ channels, which (7) creates the action potential.
There are 50–100 different types of neurotransmitters. Here we discuss briefly two major classes of neurotransmitters that are involved in time-dependent diseases of the brain: acetylcholine (Ach) and the catecholamines. Acetylcholine is found throughout the PNS and CNS. It can be inhibitory or excitatory, depending on the type of receptor found on the postsynaptic neurons. One type of acetylcholine receptor, known as a nicotinic receptor, propagates a signal by opening Na+ channels on the postsynaptic neuron. Nicotinic receptors in the brain are important in functions related to attention, learning, and memory. Degradation of neurons with nicotinic receptors is involved in the progression of Alzheimer's disease. Another type of acetylcholine receptor, known as a muscarinic receptor, uses a G-coupled mechanism to propagate the signal and is found primarily in the PNS. A decrease in the number of muscarinic receptors in the heart may be one reason for the timedependent functional decline in this organ.
The catecholamines are amines derived from catechol. There are three main types: dopamine, norepinephrine, and epinephrine. Norepinephrine and epinephrine were once thought to be a single neurotransmitter, called adrenaline. Because of this, neurons that release epinephrine or norepinephrine are called adrenergic neurons and the receptors that bind these catecholamines are called adrenergic receptors. Neurons that release norepinephrine are found primarily in brain centers that control the basic functions of life (see the discussion of the brain that follows). Epinephrine-releasing neurons are found primarily in the PNS and are responsible for our flight-or-fight response (when you jump when someone scares you, i.e., epinephrine doing its job). Dopamine-releasing neurons are primarily found in brain centers that coordinate movement. All three catecholamines can bind to two different types of receptors. The α-adrenergic receptors propagate the action potential by modulating ion channels. The ß-adrenergic receptors, like the muscarinic receptors, use the G-coupled mode of cellular signaling. As discussed in the next section, loss in function of adrenergic neurons is the primary cause of Parkinson's disease.
Human brain is collection of separate organs and cell types
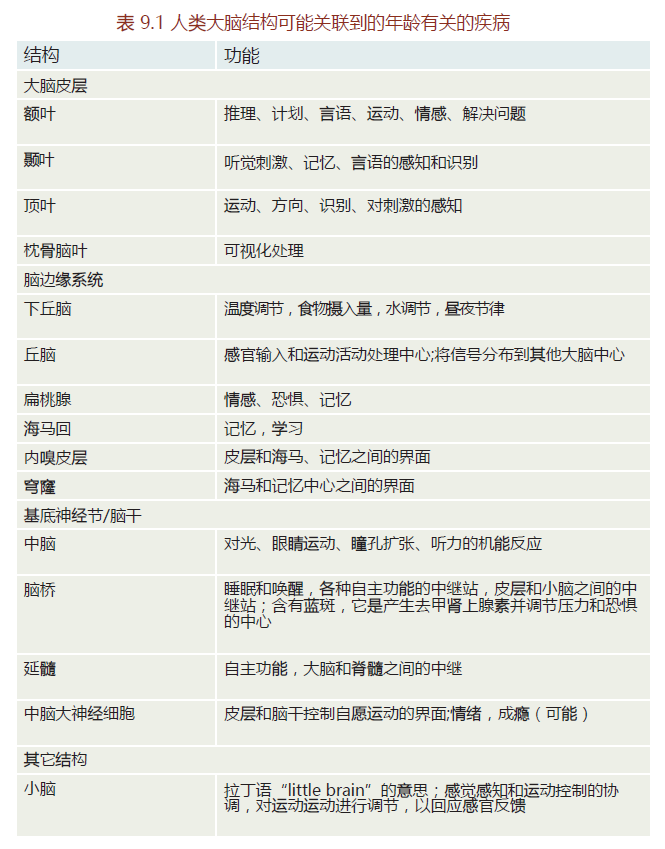
The adult human brain weighs approximately 1.3–1.5 kg. While we tend to consider the brain as one organ, it actually consists of a collection of separate neural centers, all having their own specialized function (Figure 9.6). There are hundreds of distinct neural structures and centers. TABLE 9.1 provides a list of some of the neural structures and their functions that are discussed later in connection with aging and the development of neuropathology.
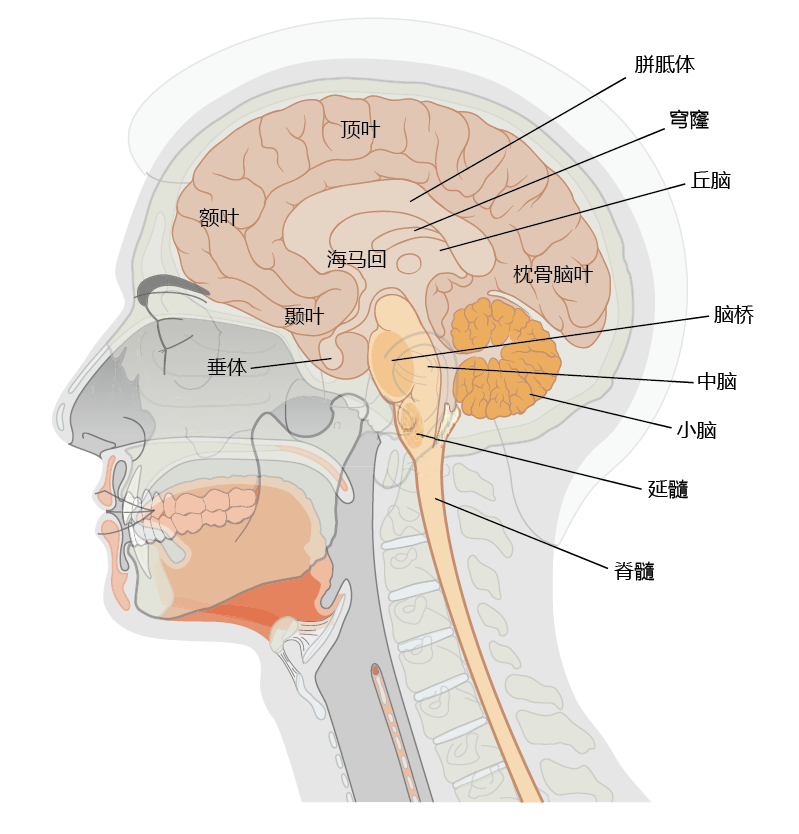
Figure 9.6 Structures of the human brain that are commonly affected by age-related pathology.
Brain tissue contains two general cell types, neurons and glial cells, or glia. Brain neurons are formed to perform a specific task at a specific location. Brain neurons are terminally differentiated and do not divide. The principle of terminal differentiation ensures that the neuron performs the task it was originally designed to carry out and will not be replaced by a different type of neuron. Although the number of neurons in the adult human brain varies among individuals, most estimates place the number at 1×1012.
The glia (Greek for “glue”), the other neurological cell type in the brain, provide support and maintenance for the neurons and outnumber neurons by about 10:1. The glial cells occur in two forms, the neuroglia and the microglia. Neuroglia can be subdivided into various cell types and classified by their function. The most abundant type of neuroglia is the astrocytes, also known as astroglia. Astrocytes maintain the proper extracellular environment for neurons by regulating ion levels and recycling neurotransmitters. Recent evidence suggests that astrocytes may also form the basis of the blood-brain barrier by regulating vasoconstriction and vasodilation. Oligodendrocytes, another type of neuroglia, produce myelin, the protein that coats axons and improves electrical conduction by providing insulation.
Microglia are mobile neuromacrophages capable of phagocytosis and initiating the inflammatory response. Although microglia make up less than 15% of all cells in the brain, they perform a critical function that may be important to aging—they respond to damage by removing injured or nonfunctional neurons. The failure to remove damaged brain tissue may establish conditions leading to various time-dependent neuropathologies.
In addition to glia, the brain is protected from insult by the blood-brain barrier, a physiological mechanism that places a barrier between circulating blood and brain tissue. This barrier alters the permeability of brain capillaries so that large blood-borne molecules or particles, such as bacteria, do not pass into the brain tissue (infections of the brain are rare). The altered permeability of brain capillaries arises from structures called tight junctions. Tight junctions increase the points of cohesion between the endothelial cells of the brain capillaries, reducing the space for diffusion through the capillary wall. Recent evidence suggests that alterations to the blood-brain barrier may contribute to the progression of Alzheimer's disease.


