9.2 TIME-DEPENDENT DISEASES OF HUMAN BRAIN: ALZHEIMER'S AND PARKINSON'S DISEASES
Compared with what scientists know about other human organs, they have only a rudimentary understanding of how the human brain performs its functions. We know even less about how aging affects brain function. Nonetheless, we do know that the healthy human brain seems to retain significant function and to show little structural change throughout most of the adult life span. In cases where changes have been reported, there is significant variation among individuals with respect to the amount of time-dependent dysfunction. Moreover, the anatomical location in the brain where time-dependent functional loss occurs also differs significantly among individuals. Thus, generalities about time-dependent functional loss in the human brain are difficult to make. You will see, however, that time-dependent alterations in the human brain, although minor, have the potential to progress to devastating neuropathology.
In this section, we focus exclusively on the two most common time-dependent diseases that affect the CNS: Alzheimer's disease and Parkinson's disease. (Time-dependent diseases of the PNS, the nerves that carry information to and from the CNS, have not been well studied and are not covered here.)
Changes in structure and neurotransmission seem to be minor in aging brain
Structural changes in the brain at advanced ages are consistently found to be minor, to vary among individuals, and to not affect the same anatomical location in all people. Moreover, the ability of the brain to develop new neural connections, (i.e., its neuroplasticity) remains remarkably robust in the aging human brain. In fact, studies using magnetic resonance imaging (MRI) find only small changes in brain size with aging. The lack of brain atrophy reflects two general observations: (1) neural cell number remains fairly constant throughout the adult life span, and (2) glial cell formation slightly increases (in a process called gliosis) with age. When neural cell loss has been reported, it appears to be limited to specific brain centers such as the hippocampus, locus coeruleus, and cerebellum. The importance of time-dependent neural cell loss to brain function remains obscure, because slight neural cell loss does not correlate well with decreased function.
There also seem to be only minor time-dependent changes in the structures involved in neurotransmission, including synaptic density, synaptic size, and volume of the synaptic cleft. Determining these values for large populations of aged individuals is technically difficult. Reports of a postreproductive decline in synaptic quality have generally been limited to brains derived from autopsy, synaptic quality appears to be highly maintained, except in the hippocampus, where synaptic size and volume decline. However, functional losses due to changes in synaptic quality have not been demonstrated.
Current evidence also suggests that concentrations of neurotransmitters, such as acetylcholine, norepinephrine, epinephrine, and dopamine, do not decrease significantly with age. However, measurements can be done only indirectly in large populations by measuring neurotransmitter concentrations in blood and urine. New techniques using labeled probes in combination with imaging technology are being introduced, but the population of aged individuals in which these techniques have been applied remains too small for definitive conclusions.
Amyloid plaques and neurofibrillary tangles accumulate in the aged brain
The aging human brain appears to have a diminished capacity to adequately rid itself of damaged proteins, and this results in an accumulation of potentially toxic compounds. Two of these neurotoxic compounds, amyloid plaques and neurofibrillary tangles, have been identified as precursors to neurological diseases such as Alzheimer's disease and Parkinson's disease (Figure 9.7). However, accumulation of these compounds in the healthy aging brain does not seem to have a significant effect on functional capacity in the majority of postreproductive individuals. Only about 10%–20% of aged individuals will experience a transition from normal aging to definable neurological disease as a result of the accumulation of amyloid plaques and neurofibrillary tangles. More than 150 years ago, the father of modern pathology, Rudolf Virchow, described a “waxy substance” in the brain of older individuals who had the appearance of a starch-like compound. He named this substance amyloid after amylum, the Latin term for “starch.” (The structure of the amyloid protein is not related to the carbohydrate family, but the name remains.) For the next 130 years, detailed descriptions of amyloid plaques were published. Then, in 1984, the amino acid sequence of the protein forming the amyloid plaques, the amyloid-beta (Aß)-protein, was elucidated. (The ß designation refers to the protein's secondary structure, a ß-sheet, a rigid structure formed by hydrogen bonding, as shown in Figure 9.8.) The amyloid fibrils (plaque) are made up of several Aß-proteins wrapped around each other to create a highly insoluble molecule. Analysis of the amino acid sequence of the Aß-protein led to the discovery of the gene that encodes the protein. The gene was found to be part of a larger gene, localized to chromosome 21, that encodes the amyloid precursor protein (APP). APP has a large hydrophilic extracellular domain, a transmembrane domain consisting of 23 amino acids, and a small intracellular domain (Figure 9.9). The expression of APP occurs in both neural and nonneural tissue. Although the function of APP has yet to be fully elucidated, physiological studies suggest that APP supports dendrite outgrowth, synaptogenesis, and inhibition of platelet activation, and it may function as a copper transport protein.
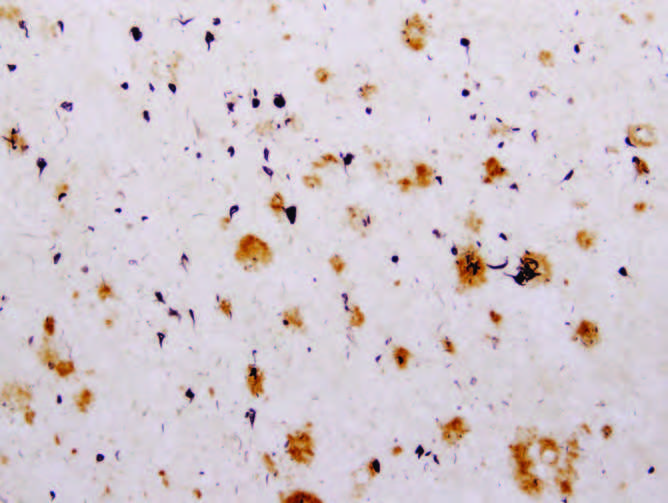
Figure 9.7 Amyloid plaques (large circles) and neurofibrillary tangles (small circles) in the brain tissues of an individual who had Alzheimer's disease. (From McGeer EG, McGeer PL. 2001. Mol Interv 1:22–29. With permission from American Society for Pharmacology and Experimental Therapeutics.)
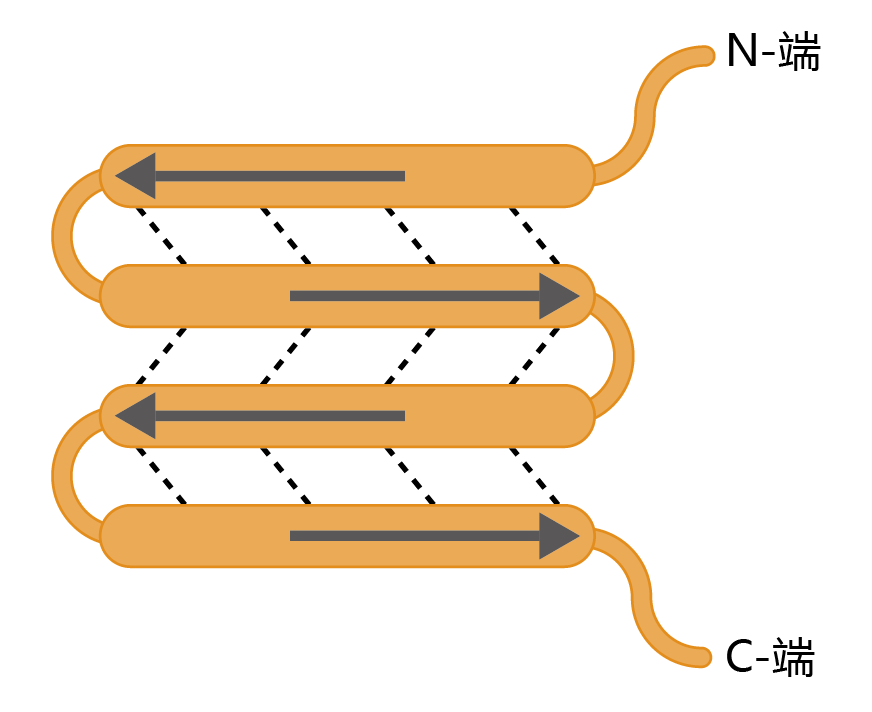
Figure 9.8 The ß-sheet secondary structure of a protein. Arrows indicate the direction (N-terminus to C-terminus) of the amino acid sequence. Dotted lines indicate the hydrogen bonds that hold the sheets together. Here we show an antiparallel pattern of the hydrogen-bonded sequences, the most common and rigid configuration of the several classes of the β-sheet motifs found in amyloid plaques.
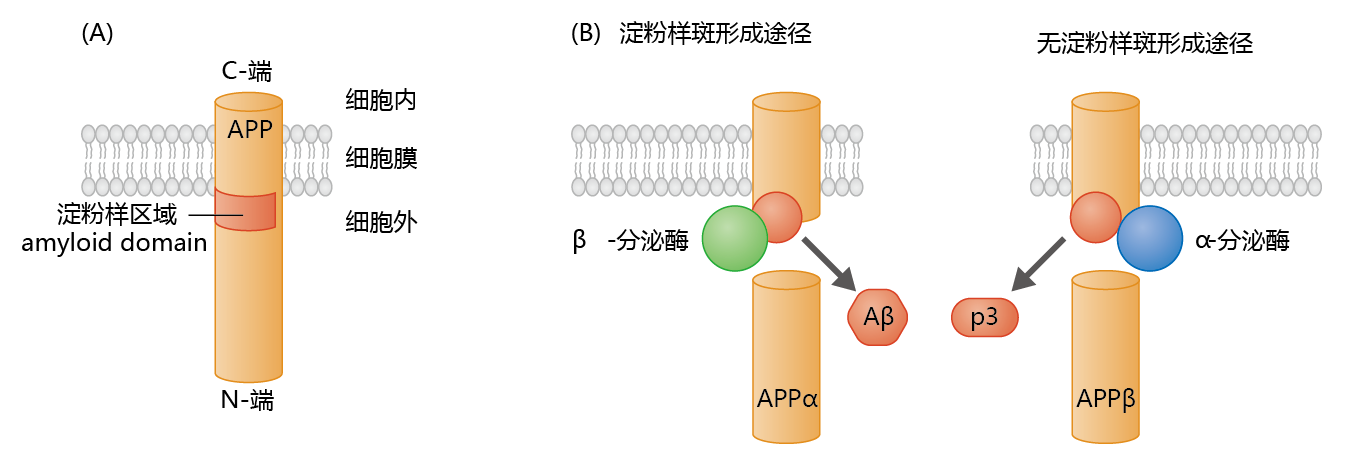
Figure 9.9 The amyloid precursor protein (APP) and formation of the ß-protein. (A) APP contains an amyloid domain (40–42 amino acid residues) that is mainly located in the extracellular space, with just a small hydrophobic region within the membrane. (B) The β-secretase, an enzyme found primarily in neural tissue, cleaves APP just below the amyloid domain. This leaves a small intramembrane protein with a conformation favoring formation of the β-protein. Cleavage of APP by α-secretase, the enzyme found primarily in nonneural tissue, favors the formation of the nontoxic p3 protein.
Synthesis of APP in neural tissue takes place in the endoplasmic reticulum, with posttranslational modification occurring during transit to its position in the cell membrane. The processing of APP within the membrane can follow one of two proteolytic pathways (Figure 9.9). The nonamyloidogenic, or α, pathway produces the protein, p3 (3 kD), whose function and metabolism remain largely unresolved. The amyloidogenic, or ß, pathway produces the Aß-protein. Enzymes known as secretases determine which proteolytic pathway a membrane-bound APP will follow.
In nonneural tissue, α-secretase predominates, resulting in the production of the nontoxic p3 protein. In contrast, in neural tissue, ß-secretase (also known as ß-site APP cleavage enzyme 1, or BACE1) predominates, resulting in formation of the potentially neurotoxic Aß-protein.
Production of Aß in neural tissue leads to the formation of amyloid plaque, a highly insoluble structure with no known catabolic pathway (Figure 9.10). The pathway from Aß to amyloid plaque has not been described in detail, although a general theory has begun to emerge. It appears that Aß polymerizes through sequential formation of dimers, tetramers, and long oligomers. The oligomers aggregate into a stacked conformation, known as a ß-cross formation, forming a fibril. Several fibrils then aggregate to form the plaque. Enzymes catalyzing Aß polymerization have not been identified, suggesting that aggregation is nonenzymatic and concentration dependent. Aß amyloid plaques are found primarily in the extracellular space, as would be predicted from the APP-processing mechanism. However, recent studies have found low concentrations of intracellular Aß amyloid plaques in the Golgi complex and endoplasmic reticulum.
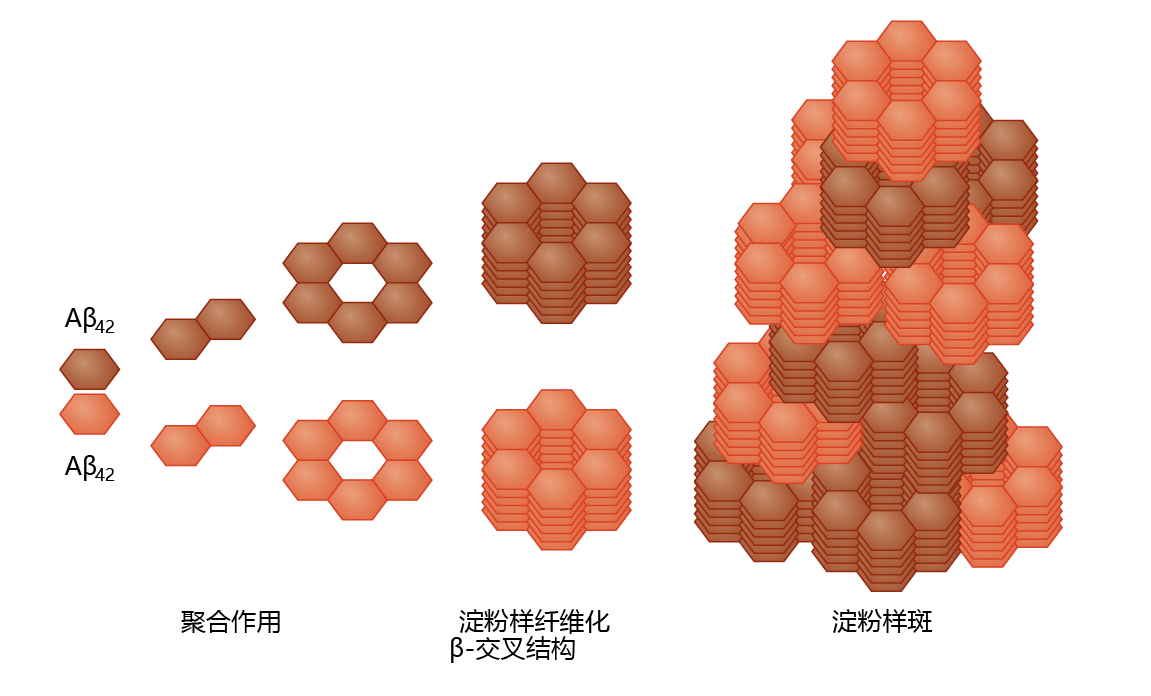
Figure 9.10 Amyloid plaque formation. Left to right: At high concentrations, the Aβ 42 protein cleaved from the APP complex (see Figure 9.9) forms aggregates by sequential polymerization. The polymerization process leads to the formation of a fibril in the β-cross configuration—a configuration that resists proteolysis. This induces further aggregation into the stacked configuration of the amyloid plaque.
The limited understanding of the mechanism underlying amyloid plaque formation reflects the unique ability of Aß to form aggregates. The formation of protein aggregates in biological systems is the exception rather than the rule and most likely reflects an abnormally folded precursor protein—in the current scenario, the Aß-protein. The cell invests considerable energy into ensuring that the folding process occurs without error and independent of other proteins, a critical control mechanism needed to maintain the structure-function relationship of protein activity. Several intracellular proteins, many of which are part of the chaperone family, support the protein-folding process by inhibiting aggregate formation or by molecularly marking for degradation those proteins that have formed an aggregate. Such chaperone proteins do not seem to exist for the Aß-protein, resulting in an aggregate—that is, the amyloid plaque—that resists proteolysis.
Neurofibrillary tangles are insoluble twisted fibers found inside brain cells. The microtubules of the cytoskeleton perform a vital function by directing the movement and determining the final position of organelles, proteins, or both, from the cell body to the axon. Under normal conditions, binding of the tau protein to the microtubule provides stability to this cellular structure (Figure 9.11). The protein's function as a support protein depends on its degree of phosphorylation, a process regulated by various kinases and phosphatases. Normal tau has 2–3 moles of phosphate per mole of protein. However, histological studies have shown that brain tissue taken from neurologically healthy aged individuals contains low concentrations of tau protein having phosphate binding at four to five times the normal level. The hyperphosphorylated tau proteins weaken the integrity of the microtubule, increase the risk of neurodegeneration, and lead to the formation of neurofibrillary tangles.
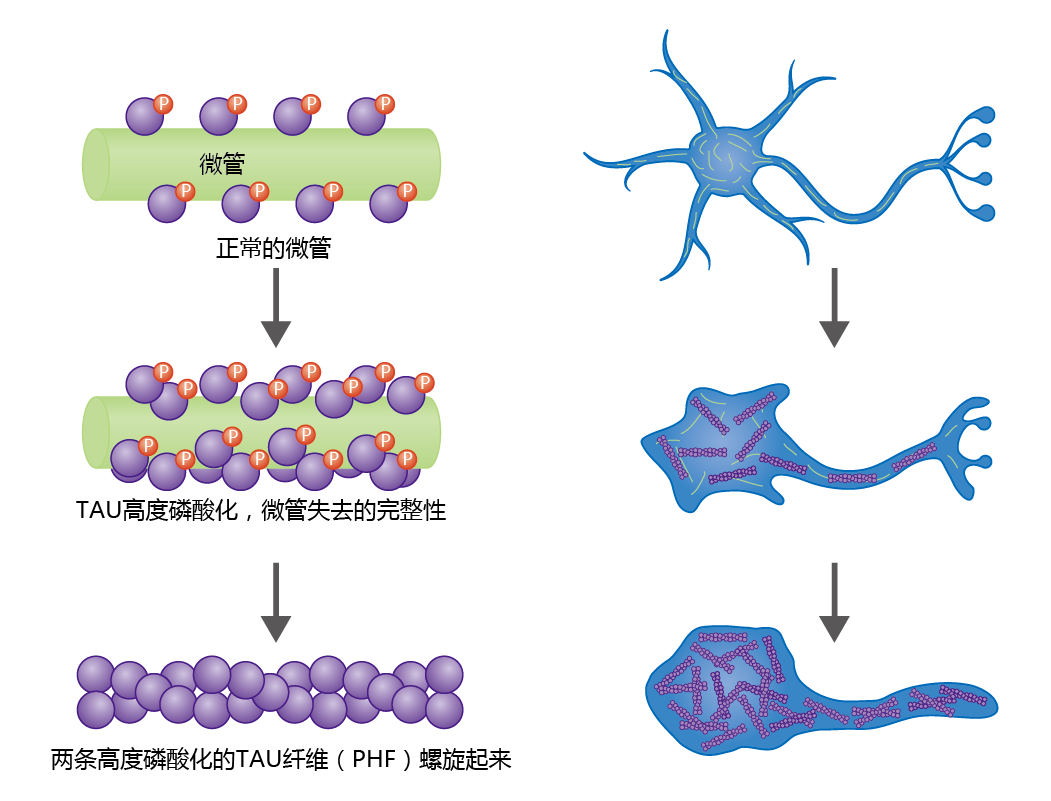
Figure 9.11 Formation of paired helical fibrils that result in neural fibrillary tangles and neural degeneration. The microtubules of the cytoskeleton maintain their integrity by means of microtubule-associated proteins (MAPs), such as the tau protein shown here. Tau proteins bind to the microtubule surface and perform their function through phosphorylation and dephosphorylation by kinases and phosphatases. If the tau proteins become hyperphosphorylated, the microtubules lose their integrity, leading to neural degeneration. Hyperphosphorylated tau proteins aggregate into insoluble structures called paired helical fibrils (PHFs), which are the primary component of neurofibrillary tangles.
Microscopic observation shows that hyperphosphorylated tau protein aggregates to form paired helical fibrils (PHFs), which aggregate further, leading to a breakdown in microtubule structure and disruption of intracellular transport. In turn, organelles important in maintaining the integrity of the neuron begin to break down, causing cell degeneration and dysfunction. Ultimately, PHFs replace the microtubule network, resulting in complete neural cell degeneration.
Paired helical fibrils are highly insoluble and cannot be degraded by the microglia, and thus they remain permanently affixed to the brain's extracellular matrix. Controversy exists about the effect of tangles on brain function. One theory suggests that tangles are inert and simply represent the end process of time-dependent neural degradation. Others suggest that tangles have a toxic effect on healthy nerve cells, which, in turn, induces further formation of PHFs.
Alzheimer's disease is a time-dependent, nonreversible brain disorder
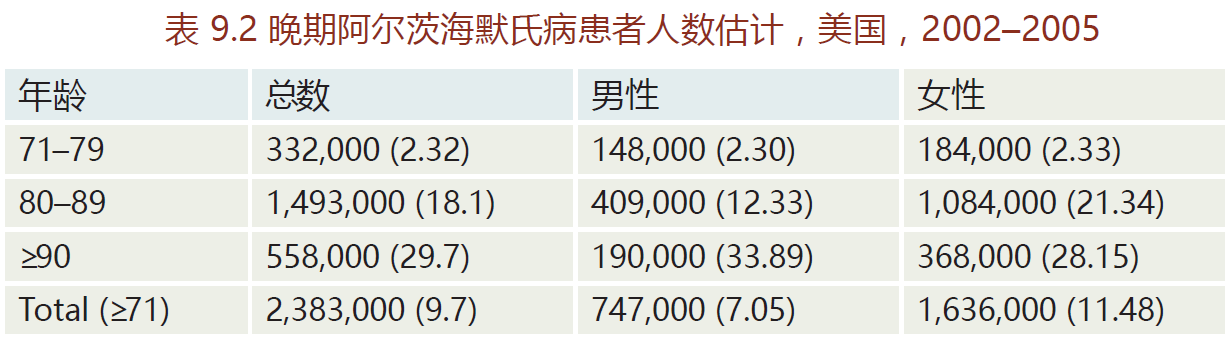
Alzheimer's disease, named after Alois Alzheimer (1864–1915), is a type of time-dependent dementia that causes problems with memory, thinking, and behavior. There are three primary types of Alzheimer's: early onset, late-onset, and familial. Early onset is a rare form of Alzheimer's in which people are diagnosed with the disease before age 65. Most cases occur in 40- to 50-year-old patients with Down's syndrome, an observation implicating the involvement of genes on chromosome 21. Early onset Alzheimer's progresses more rapidly than the other two forms and leads to brain abnormalities not observed in late-onset Alzheimer's disease. The late-onset disease, the most prevalent form (80%–90% of all Alzheimer's cases), normally begins after the age of 65 (TABLE 9.2). Whether late-onset Alzheimer's disease has a genetic component remains unknown. Most epidemiological surveys have not been able to show a significant family history for the late-onset disease. In contrast, familial Alzheimer's disease (FAD) has a direct genetic component; it accounts for less than 5% of Alzheimer's cases. The genes associated with FAD appear to be located on three chromosomes: 1, 14, and 21.
The principal features of all three types of Alzheimer's disease are the same and are widely described, but the cause appears to be multifactorial, varies with the type of disease, and remains unknown. As noted, mutations in genes on chromosomes 1, 14, and 21 have been shown to be linked to Alzheimer's disease, but they seem to directly affect only those with FAD. The mutations associated with FAD all have a role in the processing of APP and may lead to the accumulation of amyloid plaques. Neither genetics nor family history has been strongly correlated with late-onset Alzheimer's. Nonetheless, a polymorphism, an allele variant, on chromosome 19 has been identified as increasing the risk of developing Alzheimer's. Inflammation, oxidative stress, and disruption in the production of neurotransmitters have also been suggested as possible contributing factors to the etiology of Alzheimer's disease. The evidence supporting a direct link between these factors and Alzheimer's is not yet strong enough to warrant inclusion here.
Alzheimer's disease begins in entorhinal cortex and progresses into cortex
All types of Alzheimer's disease show a similar progression of symptoms and brain pathology (Figure 9.12). The early or preclinical pathology begins in the entorhinal cortex, a small structure at the base of the hippocampus that is responsible for relaying information between the cortex and hippocampus. Some estimates suggest that preclinical pathology can begin 10–15 years before symptoms appear. The mild loss of function in the hippocampus during the preclinical stage leads to some memory loss, a symptom that may be noticed only in retrospect, after diagnosis. As the disease progresses into the mild or clinical stage, cortical shrinkage occurs in the frontal, temporal, and occipital lobes. The symptoms that begin during this stage reflect effects in areas of the cortex (see Table 9.1). That is, loss of neurons in the cortical areas results in declining language skills (temporal lobe), mild loss in reasoning (frontal lobe), and hallucinations (occipital lobe). The patient also may experience changes in circadian rhythm that may lead to day-night reversal, such as sleeping during the day and being awake at night. The day-night reversal disappears in many Alzheimer's patients as the disease progresses to more advanced stages. The patient's sense of personality and relationship to the world also begins to deteriorate during the mild stage. Amyloid plaques and neurofibrillary tangles spread into the brainstem, affecting autonomic function. In particular, the locus coeruleus, located in the pons, becomes affected, causing a decline in the ability to regulate stress and panic.
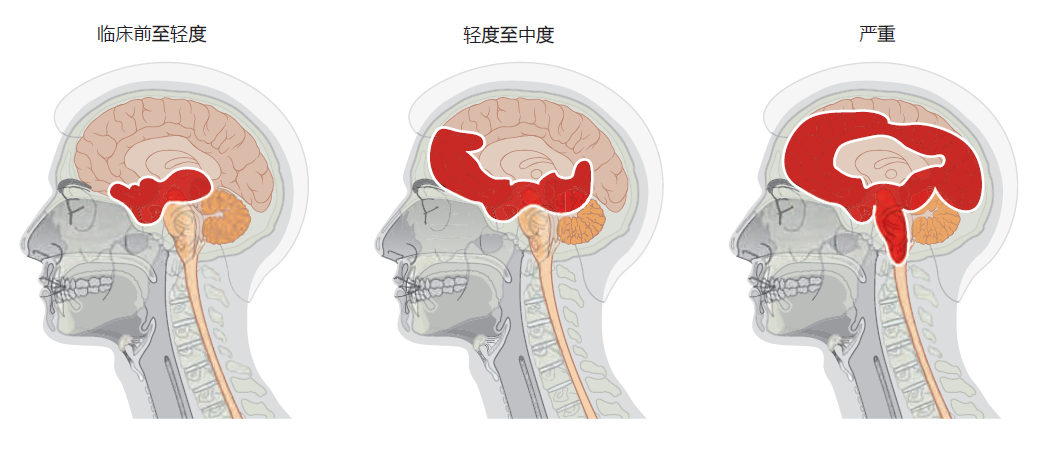
Figure 9.12 Progression of Alzheimer's disease from preclinical to severe. Alzheimer's begins subtly, and without symptoms, in the entorhinal cortex, which lies just below the hippocampus. With the transition from mild to moderate Alzheimer's disease, the hippocampus shrinks considerably and neurofibrillary tangles and amyloid plaques begin to appear in the cortex. Areas in the upper brainstem are also affected during the moderate stage. In the final stage of Alzheimer's, large areas of the cortex are involved. The disease spreads into the autonomic centers of the medulla oblongata, causing difficulties in respiration. Death due to pneumonia or heart failure is common.
In the final stage of Alzheimer's disease, also referred to as the demented stage, the hippocampus is half the size of that found in a normal brain, resulting in complete loss of memory. Severe neurodegeneration in the cortex leads to an inability to speak or understand any form of communication. The patient loses all sense of self. The cerebellum atrophies, and the patient can no longer control his or her movements, resulting in confinement to bed. In the 2–3 months prior to death, the pathology spreads into the medulla oblongata, affecting basic autonomic functions such as bowel and bladder control, heart rate, swallowing, and respiration. The patient's inability to breathe properly and expel material from the lungs often leads to pneumonia and death.
The ε4 allele of the apolipoprotein E gene is risk factor for late-onset Alzheimer's disease
Although the cause of Alzheimer's disease remains unknown, a genetic variant of the lipid-binding protein apolipoprotein E (ApoE) has been found to be more frequent in patients with late-onset Alzheimer's. The APOE gene resides on chromosome 19 and has three common alleles, ε2 (protective against Alzheimer's), ε3 (the most frequent in Caucasians), and ε4 (a risk factor for Alzheimer's). Synthesis of ApoE occurs in the liver, and its function has been primarily associated with the transport of cholesterol in the blood (the importance of this protein to cardiovascular health is discussed later in the chapter). In the brain, ApoE is synthesized by astrocytes and microglia, and here it has a major role as an extracellular lipid carrier and in the maintenance of the microvasculature.
Approximately 40%–60% of individuals who have at least one copy of the ε4 allele develop late-onset Alzheimer's disease. That is, having the ε4 allele does not cause Alzheimer's disease, it only increases the risk. The risk of developing Alzheimer's increases with the number of copies of the ε4 allele. Receiving only one copy increases the risk by 30% compared with those without this allele variant. A person who receives a copy of the ε4 allele from both parents has a 50%–60% chance of developing the disease. These risk factors are striking when compared with the 9% lifetime risk for Alzheimer's disease in people without the ε4 allele. The biochemical mechanism underlying ApoE's impact on Alzheimer's remains, by and large, a mystery.
Treatments for Alzheimer's disease target neurotransmission and prevention and degradation of amyloid plaques
In general, medications currently in use for treating patients with Alzheimer's focus on helping to delay symptoms or prevent them from becoming worse for a limited period of time. Current medications do not cure or prevent the progression of the disease. (BOX 9.1 provides information on the National Alzheimer's Project, a focused research program with the aim of overcoming Alzheimer's disease and related dementias.) The medications currently prescribed for mild to moderate Alzheimer's are known as cholinesterase inhibitors and most likely work by inhibiting the breakdown of acetylcholine, the neurotransmitter important for memory and cognition. The three most widely prescribed cholinesterase inhibitors are galantamine (Razadyne), rivastigmine (Exelon), and donepezil (Aricept). Because Alzheimer's is characterized by a progressive loss in the brain's ability to synthesize acetylcholine, cholinesterase inhibitors are effective for just a limited time. The medication used for the treatment of moderate to severe Alzheimer's is memantine (Namenda). The precise reason that memantine helps Alzheimer's patients think more clearly remains obscure, but the chemical is known to prevent accumulation of glutamate in the synaptic cleft. Glutamate, a neurotransmitter normally found in areas of the brain associated with cognition, can be toxic to neurons in abnormally high amounts.
| BOX 9.1 NATIONAL ALZHEIMER'S PROJECT ACT |
|
Alzheimer's disease burdens an increasing number of our Nation's elders and their families, and it is essential that we confront the challenge it poses to our public health. President Barack Obama, 2011 On January 4, 2011, President Barack Obama signed into law the National Alzheimer's Project Act (NAPA; https://www.congress.gov/111/plaws/publ375/PLAW-111publ375.pdf). The public law establishes the National Alzheimer's Project (NAP) and requires the Secretary of the U.S. Department of Health and Human Services (HHS) to 1. Create and maintain an integrated National Plan to overcome Alzheimer's disease and related dementias. 2. Provide information and coordination of Alzheimer's disease research and services across all federal agencies. 3. Accelerate the development of treatments that would prevent, halt, or reverse the course of Alzheimer's disease. 4. Improve the early diagnosis of Alzheimer's disease, and coordinate the care and treatment of citizens with Alzheimer's. 5. Ensure the inclusion of ethnic and racial populations at higher risk for Alzheimer's or least likely to receive care, in clinical, research, and service efforts with the purpose of decreasing health disparities in Alzheimer's. 6. Coordinate with international bodies to integrate and inform the fight against Alzheimer's globally. The act instructs the Secretary of HHS to establish a diverse advisory council that is responsible for developing a plan to overcome Alzheimer's disease and related dementias. The members of the advisory council include individuals from several federal agencies such as the U.S. Food and Drug Administration, U.S. Department of veterans Affairs, and the Centers for Disease Control and Prevention. The advisory council also includes 12 members from outside the federal government with expertise in Alzheimer's advocacy, caregiving, and biomedical research. The composition of the advisory council was establish to optimize the existing federal resources and encourage cooperation between the public and private sectors in creating a new way of treating and preventing Alzheimer's disease. In May 2012, the Advisory Council unveiled the first National Plan to Address Alzheimer's Disease. The plan established the first goal of the National Plan, Goal 1: To prevent and effectively treat Alzheimer's Disease by 2025. Since that first goal, four additional goals have been added to the National Plan to Address Alzheimer's Disease: Goal 2: Enhance Care Quality and Efficiency Goal 3: Expand Supports for People with Alzheimer's Disease and Related Dementias and Their Families Goal 4: Enhance Public Awareness and Engagement Goal 5: Improve Data to Track Progress The Advisory Council provides HHS yearly with a report outlining progress made on the five goals. Although the progress reports are too detailed to include here, significant progress has been made to all goals. One of the more important steps toward achieving the five goals has been the establishment of 31 Alzheimer's Disease Research Centers throughout the country. Areas of investigation range from the basic mechanisms of Alzheimer's to managing the symptoms and helping families cope with the effects of the disease. Center staff conduct basic, clinical, and behavioral research and train scientists and health-care providers. Although each center has its own area of emphasis, a common goal of the ADCs is to enhance research on Alzheimer's disease by creating a network that shares new ideas and research results. Collaborative studies draw on the expertise of scientists from many different disciplines. Additional information on the National Plan to Address Alzheimer's Disease can be found at the following websites: National Institute on Aging, Alzheimer's Disease Education and Referral Center: https://www.nia.nih.gov/alzheimers 2016 Update on the National Plan to Address Alzheimer's Disease: https://aspe.hhs.gov/report/national-plan-address-alzheimers-dis-ease-2016-update U.S. Department of Health and Human Services, National Alzheimer's Project Act: https://aspe.hhs.gov/national-alzheimers-project-act |
Ongoing research into new treatments and therapies for Alzheimer's disease has focused primarily on preventing the formation of protein aggregates or degrading them once they have formed. One hypothesis posits that plaque formation may be associated with altered immune function. This hypothesis was tested by injecting antibodies to Aß-protein into transgenic mice that overexpress Aß-protein and accumulate amyloid plaques. Remarkably, the plaque concentration decreased significantly in mice receiving the antibodies compared with those receiving a placebo injection. Clinical trials in humans using a similar approach are currently underway.
Some of the most recent research has linked two important questions concerning the development of Alzheimer's disease: (1) Why do all aging brains show some accumulation of amyloid plaques, but only a smaller percentage experience the widespread accumulation associated with Alzheimer's? (2) What is the mechanism underlying the increased risk of Alzheimer's associated with the APOE ε4 (APOE4) gene variant? The answers to these questions may lie in an abnormal disruption in the blood-brain barrier. Recall that the blood-brain barrier protects the brain by preventing the entry of large molecules. We also know that the most common APOE variant, APOE2, expresses a protein that supports vessel integrity, whereas the protein expressed by the APOE4 variant seems to injure capillary cells. Using this knowledge, researchers found that transgenic mice overexpressing APOE4 showed increased damage to cells of the tight junctions that form the blood-brain barrier when compared with APOE2 transgenic mice. The resulting damage would cause holes in the tight junctions and allow large molecules in the blood, such as the Aß-protein, to breach the blood-brain barrier. If correct, these findings would help to solve the mystery of why excess amyloid plaque accumulation occurs in Alzheimer's disease but not in normal aging. Most important, this research found that the damage caused by the protein product of APOE4 responded to pharmacological treatment in the transgenic mice, suggesting that similar treatments could be developed for humans.
Effective treatments for Alzheimer's disease will require reliable biomarkers
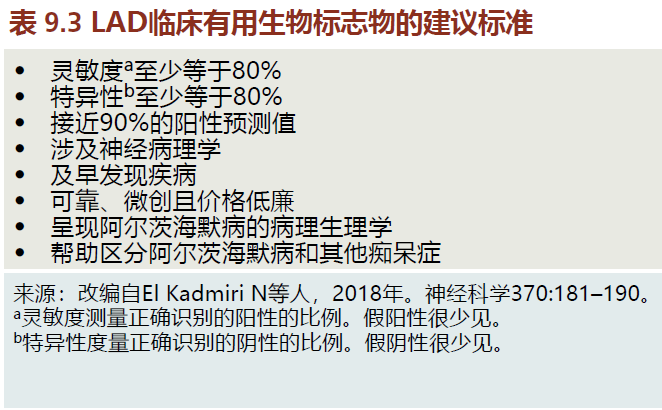
You learned that the degenerative process of late-onset Alzheimer's disease (LAD) begins many years before the symptoms appear. Once the clinical signs and symptoms of LAD are present, damage to the neural tissue causing cognitive impairment is too extensive for treatments to be effective. The most effective treatments will be those that attack the disease during its early stages, long before the clinical signs and symptoms are seen (known as the preclinical stage of LAD). Detecting LAD in the preclinical stage will require clinically reliable and valid biomarkers. Although current biomarkers for LAD have not yet achieved the suggested criteria for use in the clinical setting (TABLE 9.3), research funding for this area has been given the highest priority. Nonetheless, many of the biomarkers described in the following sections are being used in the research setting to confirm the neuropsychological diagnosis of LAD (see the following text).
Much of the initial research into LAD body fluid biomarkers has focused on detection of Aβ and tau proteins in cerebral spinal fluid (CSF). Numerous investigations have shown that the concentration of Aβ 42 in CSF declines by about 50% in LAD patients versus similarly aged nondemented individuals. Autopsy studies report that the greater the amyloid plaques in the brain, the lower is the Aβ 42 concentration. The inverse relationship between Aβ 42 concentration in the CSF and the degree of amyloid plaques seen in the LAD brains seems counterintuitive and has yet to be fully explained. We do know that the soluble form of the Aβ protein, Aβ 40, remains stable in the CSF in patients with LAD. As such, recent research has shown that the ratio of Aβ 42 :Aβ 40 in the CSF may provide a better biomarker for LAD than does Aβ 42 alone.
The total concentration of the tau protein (t-tau) and the hyperphosphorated tau protein (p-tau) have also shown promise as biomarkers. Cerebral spinal fluid t-tau can indicate transition from mild cognitive impairment (MCI), a clinical predictor of LAD (see the following text), to full-blown LAD. However, the power to predict LAD from t-tau in CSF in patients with MCI is only about 50%. This modest predictive power most likely reflects the fact that several isoforms of the tau protein make up the t-tau value, and only one or a few of these isoforms are specific to LAD. For tau to be a specific and reliable predictor for LAD, the isoforms most associated with the disease must be identified. The hyperphosphorated tau protein may be a better predictor of LAD, since the tau isoforms do not impact the assay. Indeed, some studies have shown 90%–95% of individuals with MCI and high level of CSF p-tau progress into LAD.
The major drawback in using CSF Aβ and tau proteins as biomarkers for LAD lies in the technique itself. Drawing fluid from the spinal cavity is moderately risky, often painful, and expensive, all factors that render this technique clinically inappropriate. In fact, the guidelines put forth by the National Institutes of Health (NIH) limit the use of CSF Aβ and tau proteins to research investigations. Elevated blood levels of Aβ 42 have long been known to be associated with LAD. Since Aβ 42 is generated in other tissue beside brain, the use of this protein as a biomarker for Alzheimer's shows low prediction, sensitivity, and specificity. Recent research (2017) has suggested that the validity and reliability of blood Aβ as a biomarker can be improved. These new methods will require extensive testing before being brought into the clinical setting.
Brain imaging techniques serve as biomarkers for LAD
Magnetic resonance imaging (MRI) and positron emission topography (PET) are allowing researchers and clinicians to visualize possible biomarkers for LAD. Although MRI and PET techniques have not reached the reliability and validity necessary for a true biomarker (Table 9.3), these two imaging platforms are being used in research settings to confirm a diagnosis of LAD.
Two types of MRI, functional MRI (fMRI) and structural MRI (sMRI), are used to identify possible biomarkers for LAD. Functional MRI measures the amount of blood flow to areas of the brain and is an extremely sensitive and specific indirect measurement of neuronal energy metabolism. Neuronal energy metabolism has been shown to be highly correlated with neuron density and function. Thus, a decrease in blood flow to areas of the brain most associated with LAD suggests the possibility of neuronal loss and the presence of the disease (Figure 9.13). Structural MRI allows for the visualization of brain volume. Atrophy in the hippocampus and other brain areas often associated with LAD indicates the presence of the disease (Figure 9.14). This technique has proven to be extremely sensitive and specific (see Table 9.3 for definition of sensitivity and specificity). For example, a 25% reduction in the hippocampus volume as compared to a normal individual predicts with 90% accuracy the progression of MCI to LAD.
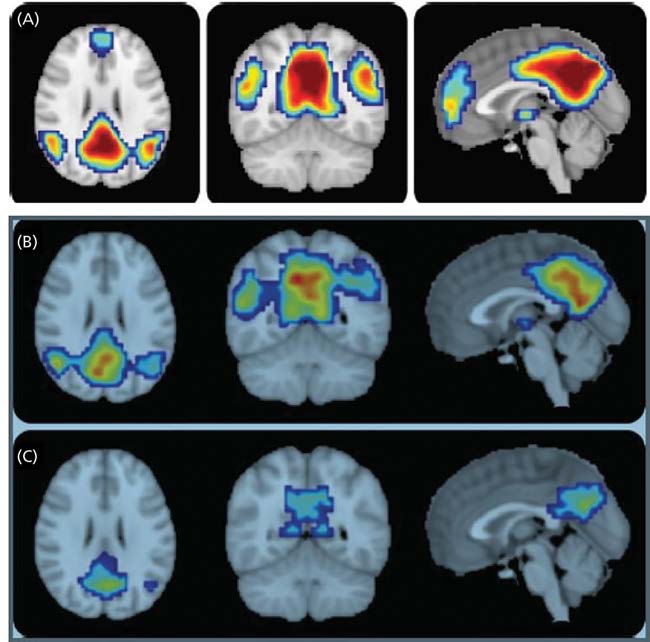
Figure 9.13 Functional magnetic resonance imaging (fMRI) taken from individuals with (A) normal cognitive function, (B) mild cognitive impairment that will not progress to LAD, and (C) mild cognitive impairment that will progress to LAD. The colors indicate the degree of blood to the posterior cingulate/precuneus and bilateral inferior parietal lobules, areas of the brain in which decreased blood flow has been associated with LAD. Reds indicate high blood flow; blues and greens are low blood flow. Note the difference between blood flows in the scans of B and C. Although the scan in B shows decreased blood flow compared to A, they are significantly greater than those of C. While the patient in B has MCI, it did not progress to LAD. This comparison allows the clinician to rule out LAD and focus on other causes responsible for the MCI. (Adapted from Petrella JR et al. 2011. Neurology 76[6]:511–517. Figures 1 and 3.)
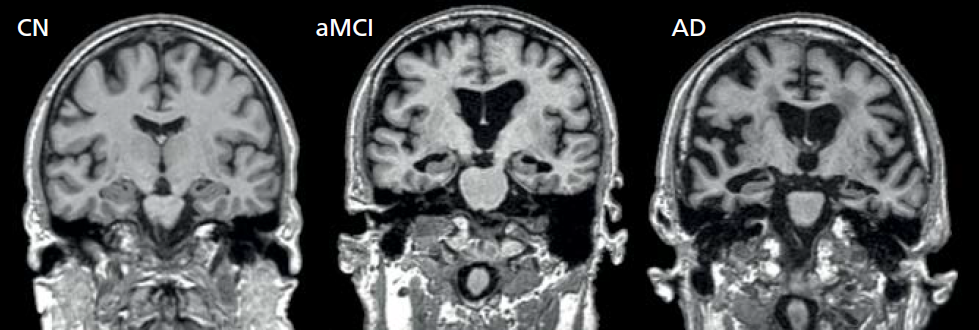
Figure 9.14 Structural MRI scans of individuals with normal cognitive function (CN), one diagnosied with mild cognitive impairment (MCI), and one with late-onset Alzheimer's disease (LAD). (Adapted from Vemuri P, Jack CR. 2010. Alzheimers Res Ther 2:23.)
Cerebral energy metabolism, a measure of neural density and function, can also be measured using PET. This technique uses glucose molecules labeled with a radioisotope, Fluorine-18, injected into the patient just prior to a PET scan. The metabolism of FDG is identical to that of glucose without a Fluorine-18 label. Thus, the positron emission associated with the Fluorine-18 can then be visualized when the FDG binds to the cell membrane on the neuron. Since the brain uses only glucose for energy production, areas showing low binding of FDG compared to normal subjects reflect a decrease in neural density and function. The prediction that MCI will progress to LAD is about 80% accurate.
One of the more promising areas of brain imaging biomarker research lies in the ability to visualize amyloid plaques and tau tangles in the brain. Techniques currently being tested work much like PET-FDG except that the radiolabeled tracer binds to amyloid plaques or tau tangles. Some research has suggested the compounds used to identify amyloid plaques or tau proteins will become sensitive enough to detect buildup plaques in the preclinical state of LAD. However, the prediction that MCI will progress to LAD in these techniques remains quite low, currently standing at about 45%–55%. Most researchers believe that prediction rate will markedly increase in the coming years with the introduction of new PET technology.
Early diagnosis of LAD focuses on detection of MCI and elimination of other dementias
You have learned that current biomarkers are not yet sensitive enough to detect LAD at the preclinical stage. The earliest diagnosis of LAD occurs at the MCI stage (see Figure 9.14). Diagnosis of MCI is made when the person presents the four criteria established by the NIH:
1. Concern regarding a change in cognition: This concern can be obtained from the patient or a person who knows the patient well.
2. Impairment in one or more cognitive functions: Impairment may include changes to memory, problem-solving, attention span, etc. There are several valid cognitive tests available to the clinician.
3. Preservation of independence in functional abilities: While the persons with MCI may have dysfunction in one or more cognitive functions, these problems should not disrupt their ability to independently carry out daily activities.
4. Not demented: The cognitive changes should be sufficiently mild such that there is no evidence of dementia—the loss of cognitive functioning such as thinking, remembering, and reasoning.
When a patient fulfills the four criteria for MCI, the health-care worker can request further testing using one or more of the biomarkers previously discussed to confirm or eliminate a diagnosis of LAD. Eliminating LAD is an important step, as other dementias such as Lewy body disease/dementia (see section later in chapter titled, “Lewy bodies are pathological hallmark of Parkinson's disease”) and vascular disease can cause MCI. Unlike LAD, other dementia may have treatments. Confirming the diagnosis of LAD in a patient diagnosed with MCI often begins when a physician requests one of many imaging options to better visualize areas of the brain. The loss of blood flow (Figure 9.13) shown through fMRI or buildup of amyloid and tau proteins plaque would be consistent with LAD. However, these same conditions can also be visualized in other types of dementia. To confirm that a patient may progress to LAD, the physician will then request a CSF analysis for Aβ and tau proteins. A decrease in CSF Aβ or an increase in p-tau in addition to evidence from the MRI or PET scan significantly increases the likelihood that MCI will progress in LAD.
Our example reflects the best-case scenario for the diagnosis of LAD from MCI. These steps are, in reality, used primarily when the individual is part of a research project or a clinical trial, or other mediating factors are observed. Although the NIH has endorsed the use of these biomarkers during the MCI phase, they remain far too expensive for general use in the clinical setting. Nonetheless, the use of biomarkers to diagnosis LAD as previously described represents a major leap forward in the fight against this disease. Understanding what biological factors correlate well with the pathology of LAD provides other researchers with a clearer focus in developing pharmaceuticals and other therapies that eliminate or delay the disease progression. In time, not only will the technology exist to detect LAD during the preclinical stage, but drugs and gene therapies will exist to treat this devastating disease.
Parkinson's disease is associated with loss of dopaminergic neurons
Parkinson's disease is a time-dependent, motor system disorder that usually affects people over the age of 50. The primary symptoms of Parkinson's are tremor or trembling of the hands, arms, legs, jaw, and face; bradykinesia, or slowness of movement; muscular rigidity; and postural instability. These symptoms reflect the loss of dopamineproducing neurons in the substantia nigra region of the basal ganglia (Figure 9.15). Patients often experience weakness and tremor on one side of the body only. Moreover, some individuals notice a “feeling of tremor” within the deep portions of large muscle groups. Tremor becomes greater during times of strong emotions, such as sexual arousal or anxiety, but subsides after the emotion has passed and returns to its normal level. An inability to create facial expressions that match the person's emotional state (smile, frown, etc.) begins to appear during the early stages of Parkinson's disease, as does a slight change in the voice. Both effects are caused by muscular rigidity and slowing of contraction speed in muscles in the head and neck. A slight stooped posture occurring during the later stage of early Parkinson's results in a feeling of being out of balance and creates difficulty in walking.
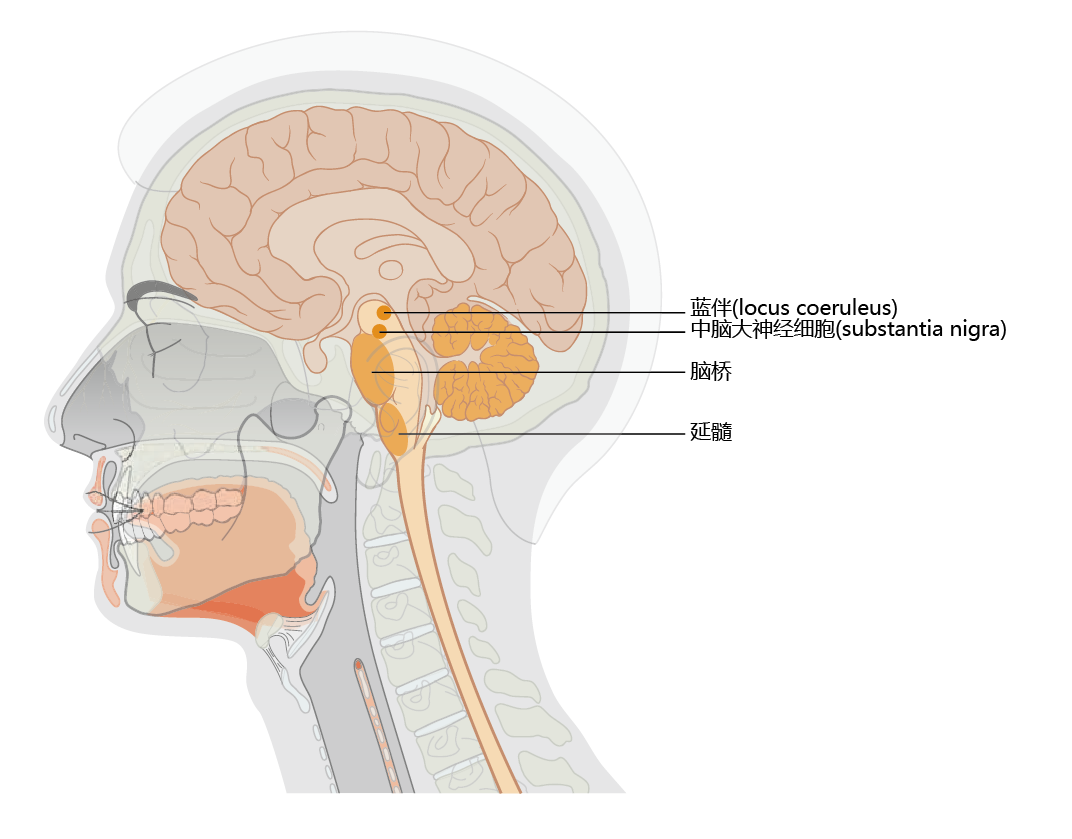
Figure 9.15 Location of the substantia nigra and locus coeruleus. Note the close proximity of the locus coeruleus to the substantia nigra. Declining synthesis of the neurotransmitter dopamine in the substantia nigra results in the early symptoms of Parkinson's disease. Loss of norepinephrine-producing neurons in the locus coeruleus occurs in Alzheimer's disease. Many Alzheimer's patients develop Parkinson's disease, and many Parkinson's patients develop Alzheimer's disease.
As Parkinson's disease progresses to the moderate stage, the motor function impairments become more severe. The muscle rigidity and cramps that accompany loss of motor function can cause considerable pain. While many of the effects caused by the declining ability of neurons in the substantia nigra to synthesize dopamine can be significantly reduced through drug therapy, the autonomic centers in the brainstem that synthesize acetylcholine, norepinephrine, or both begin to show dysfunction. Parkinson's patients often suffer from constipation, overproduction of saliva (drooling), lack of bladder control, and an inability to regulate body temperature. Finally, some individuals might suffer from what clinicians call day-night reversal. That is, the patient is awake during the night and sleeps during the day.
Although large-scale epidemiological investigations of Parkinson's disease have not been completed, estimates from several small population studies indicate that the prevalence of Parkinson's worldwide increases after age 50. Indeed, age remains the only widely accepted risk factor for Parkinson's. Occurrence of the disease in people under 50 years of age reflects a rare genetic/family form of Parkinson's that is caused by mutations in genes found primarily on chromosomes 1, 4, and 6. Controversy exists as to the variation of disease prevalence with respect to ethnicity/race and gender. For example, African Americans seem to have the highest rate of Parkinson's disease in the United States. However, the prevalence of Parkinson's in Nigeria is among the lowest in the world, suggesting that factors other than race account for the high rate of Parkinson's in African Americans. No clear difference has been shown in the prevalence of Parkinson's in men and women of similar age.
Increasing brain's concentration of dopamine is primary objective in treatment of Parkinson's disease
The cause of Parkinson's disease is loss of the neurotransmitter dopamine, and there are no treatments for reversing or curing this loss. Increasing the level of dopamine in the brain has proved effective in reducing the symptoms of Parkinson's, and there are two general approaches to this treatment. During the early stages of the disease, many physicians prescribe drugs known as dopamine agonists, which stimulate the receptors in nerves that are normally stimulated by dopamine. These drugs work only during the early stages of the disease, when there are still sufficient numbers of dopaminergic receptors/nerves to stimulate. As the disease progresses and dopaminergic receptors decline, the effectiveness of dopamine agonists declines. At this point, the focus of drug therapy shifts to increasing the concentration of dopamine in the brain. However, dopamine cannot pass through the blood-brain barrier. A precursor to dopamine, called levodopa or L-dopa, can cross the blood-brain barrier and is converted to dopamine in the brain, through a reaction catalyzed by DOPA decarboxylase. This reaction also occurs in the peripheral nervous system, however, resulting in unwanted side effects such as nausea and vomiting. In most cases, L-dopa is given together with a peripheral DOPA decarboxylase inhibitor known as carbidopa.
While the L-dopa–carbidopa combination is extremely effective at reducing the symptoms associated with Parkinson's disease, these drugs do have limits and side effects that are progressive. Among the most frequent side effects are jerky movements such as facial grimacing, postural tics, and exaggerated chewing. Long-term administration of L-dopa–carbidopa often results in low blood pressure, skin rashes, depression, and alterations in sleep patterns. The effectiveness of L-dopa–carbidopa diminishes as Parkinson's progresses and the number of dopaminergic receptors/nerves decreases.
Lewy bodies are pathological hallmark of Parkinson's disease
The cause of Parkinson's disease remains unknown. Early diagnosis is difficult, due in large part to the slow progress of the disease and clinical signs that can be similar to those of other neurological disorders. We have discussed how the accumulation of damaged or misfolded proteins in brain tissue is often associated with aging and time-dependent disease. Such proteins are also found in association with Parkinson's disease. These proteins, known as Lewy bodies, are highly insoluble aggregates of fibrous proteins that appear in the cytoplasm of neurons. Although Lewy bodies can be found in aged humans without Parkinson's, their accumulation in the substantia nigra and locus coeruleus is a pathological hallmark of the disease. The major components of Lewy bodies are two proteins that normally participate in the maintenance of protein structure, ubiquitin and α-synuclein. Ubiquitin, a small (76 amino acid) heat shock protein, attaches to misfolded or damaged proteins to mark them for degradation. The precise function of α-synuclein remains unclear, but most researchers agree that this protein plays a vital role in the maintenance and regulation of dopamine vesicles at the synaptic terminals. Its ß-sheet conformation involving specific amino acid residues also promotes the formation of ß-sheet aggregates, if the protein does not undergo degradation. These aggregates form the primary component of Lewy bodies.
Several genes are associated with early onset Parkinson's disease
Early onset Parkinson's disease, occurring before the age of 50, accounts for less than 1% of all Parkinson's cases and may be directly linked to family history and mutations in several genes. Mutations linked to early onset Parkinson's have been discovered at several loci, but genes associated with the proteins that constitute the majority of Lewy bodies, ubiquitin (the Parkin gene, on chromosome 6) and α-synuclein (a gene on chromosome 4), have drawn the most attention. The protein product of the Parkin gene functions as a component of ubiquitin ligase, the enzyme that adds ubiquitin to other proteins. A mutation in the Parkin gene disrupts the normal pathway that marks proteins for degradation, thereby allowing damaged or misfolded proteins to accumulate and protein aggregates to form. Interestingly, the primary protein aggregates found in Parkinson's disease, the Lewy bodies, are not found in the neurons of patients with the Parkin gene mutation. Rather, current research suggests that the mutation in the Parkin gene causes mitochondrial dysfunction and over-production of reactive oxygen species, leading to cell death.
Regardless of the type of mutation, in the Parkin gene or in the α-synuclein gene, only about 50% of cases of early onset Parkinson's disease can be linked to these mutations. Moreover, people with the late-onset disease, the type accounting for 99% of all cases of Parkinson's, rarely have this mutation.
Several factors may predispose individuals to Parkinson's disease
Parkinson's disease, as we have seen, does not have a clear and unequivocal cause. It is a multifactorial disorder involving both genetic and environmental factors. Mutations of genes leading to early onset Parkinson's strongly suggest a genetic component. The first clue that the environment may contribute to the disease came when drug users, in an attempt to synthesize the morphine-like drug meperidine, instead produced a neurotoxin, methyl-phenyl-tetrahydropyridine (MPTP). MPTP selectively kills dopaminergic neurons in the substantia nigra and other parts of the brainstem. Thus, when these drug users took what they believed to be meperidine, they developed an irreversible Parkinson's-like condition. Subsequent studies investigating the connection between MPTP and Parkinson's suggested that this compound inhibits a biochemical pathway in the mitochondria that protects neurons from oxidative damage. While the association between oxidative damage and Parkinson's remains only correlative, exposure to other agents that increase oxidative stress, such as Paraquat, high levels of iron and manganese, and repetitive brain trauma, has been shown to increase the risk of developing Parkinson's.
Deep brain stimulation can help control movement disorders associated with Parkinson's disease
Tremors, dystonia, and other Parkinson's disease–associated movement disorders are thought to be caused by a disruption in the neural circuitry within the subthalamic nucleus (STN) of the basal ganglia. It has been shown that electrically stimulating the subthalamic nucleus significantly reduces the motor dysfunction associated with Parkinson's disease. In order to electrically stimulate the subthalamic nucleus, a surgeon implants electrodes into the STN of the left and right hemispheres (Figure 9.16). The surgeon uses anatomical landmarks provided by an MRI to locate the STN and insert the electrode through the skull. This procedure does not require exposing the brain, and the patient remains awake during the procedure. Once the electrodes are in place and operating correctly, the patient has a second surgery under general anesthesia to connect the lead wire to a controller and battery pack implanted subcutaneously in the chest wall. After the patient has recovered from surgery, a neurologist programs the controller to deliver an electrical impulse that has the correct intensity and duration to reduce the Parkinson's disease–associated movement disorders.
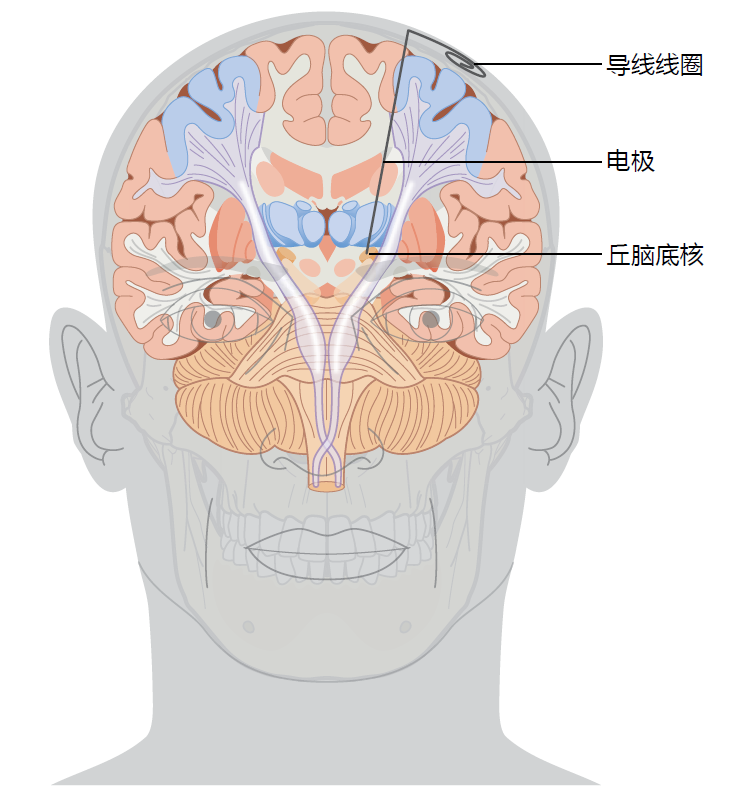
Figure 9.16 Location of the deep brain stimulus electrode. The coiled lead wires will be connected to a controller and battery pack placed subcutaneously in the patient's chest wall. (https://www.mayfieldclinic.com/PE-DBS.htm)
The reasons DBS reduces the tremors and other Parkinson's disease–associated movement disorders remain unknown. DBS does not cure the underlying mechanisms causing the Parkinson's disease–associated movement disorders. And, since Parkinson's disease is a progressive disease, the effects of DBS decline over time, although the duration and intensity of the electrical pulse can be changed to reduce the increasing tremors. Furthermore, not all patients with Parkinson's disease are candidates for DBS. The typical candidates for DBS are those whose medication has either totally lost its effectiveness or wears off before the next dose can be taken.


