9.5 ENDOCRINE SYSTEM AND GLUCOSE REGULATION
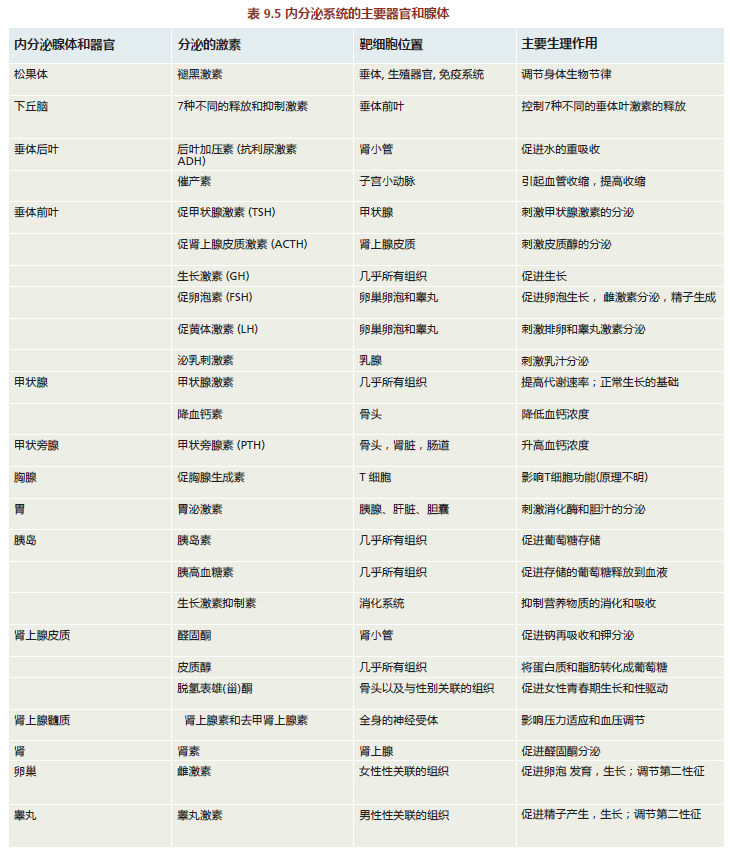
The endocrine system is a system of anatomically nonconnected organs and glands, each of which secretes hormones directly into the blood (Figure 9.29). The hormones have specific target cells, and the response of these cells to a particular hormone causes the tissue to perform specific functions (TABLE 9.6). The endocrine system is responsible for regulating many human functions, including metabolism, growth and development, various tissue functions, and maintenance of blood glucose levels. In addition to the traditional endocrine organs and glands illustrated in Figure 9.29, several others with mixed functions are now recognized as part of the endocrine system. These include the liver, heart, small intestine, adipose tissue, and skin.
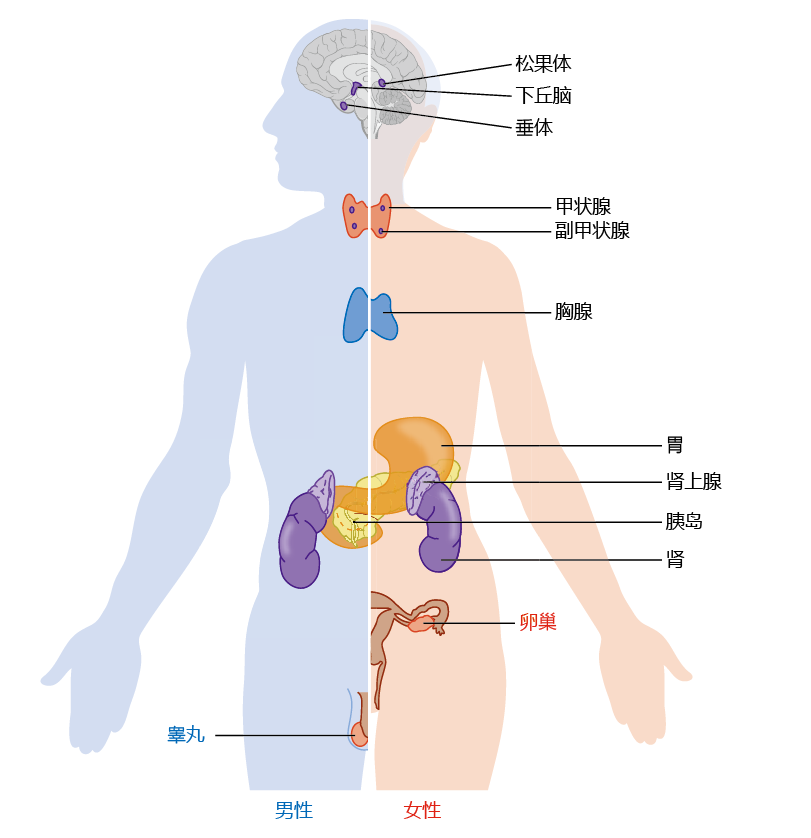
Figure 9.29 Major organs and glands of the endocrine system.
Animal studies have shown a time-dependent decline in function in virtually all endocrine organs and glands. The results from highly controlled experiments using laboratory animals, however, have not generally been corroborated in human trials. The reasons for the different findings in experimental animals and in humans are the same as those we have discussed previously: an inability to control for environmental influences in human studies, inclusion of individuals with disease in the sample population, the small number of studies, the small number of individuals included in each study, intrinsic genetic differences among species, and so on. We therefore limit our discussion here to two endocrine systems—the regulation of blood glucose and of bone calcium—for which there are sufficient data to suggest that time-dependent changes often progress to disease. In this and several following sections, we discuss the biochemical and physiological mechanisms involved in regulating blood glucose and bone calcium and the two diseases associated with altered regulation: type 2 diabetes (a disorder of blood glucose regulation) and osteoporosis (a disorder of bone calcium regulation).
Blood glucose concentration must be maintained within narrow range
You saw in earlier chapters that glucose contributes significantly to the energy needs of the body. Most tissues derive the glucose needed for energy production from their intracellular stores of glycogen. The brain, however, can only use glucose derived from the blood. The brain does not have the capacity to store glycogen and cannot easily metabolize fat for energy. Thus, the concentration of glucose in the blood must be maintained at levels that ensure a consistent supply of glucose to the brain, normally between 90 and 120 mg/dL.
The mechanism for maintaining blood glucose within the narrow range of 90–120 mg/dL involves the competitive action of insulin and glucagon, two hormones produced by the pancreas. The endocrine cells of the pancreas are localized in the islets of Langerhans (Figure 9.30). Insulin facilitates removal of glucose from the blood when the glucose concentration exceeds ∼120 mg/dL and stimulates glycogenesis (glycogen synthesis) in the liver and muscle. Conversely, glucagon facilitates addition of glucose to the blood by stimulating the breakdown of glycogen to glucose in the liver when the blood glucose concentration falls below ∼90 mg/dL (glycogenolysis). However, the human body has only a 24-hour supply of glycogen that can be used for energy. Glucose must be supplied by food to replace the glycogen stores broken down and used for energy between meals. Gluconeogenesis, the synthesis of glucose from noncarbohydrate carbon sources, does occur in mammals, but only during extended periods of starvation.
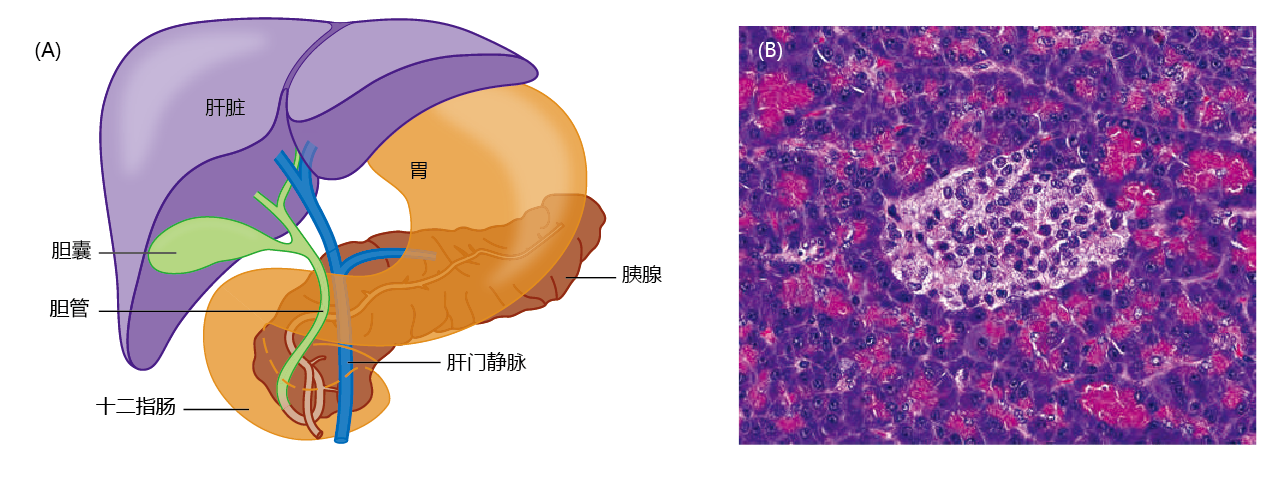
Figure 9.30 The pancreas and islets of Langerhans. (A) The anatomical location of the pancreas. (B) Micrograph of pancreatic tissue. The blue structures surrounding the islets of Langerhans (lighter area) are pancreatic acini, cells that secrete digestive enzymes into the small intestine. (B, courtesy of vetpathologist/Shutterstock.)
Insulin facilitates glucose uptake into liver, muscle, and adipose cells
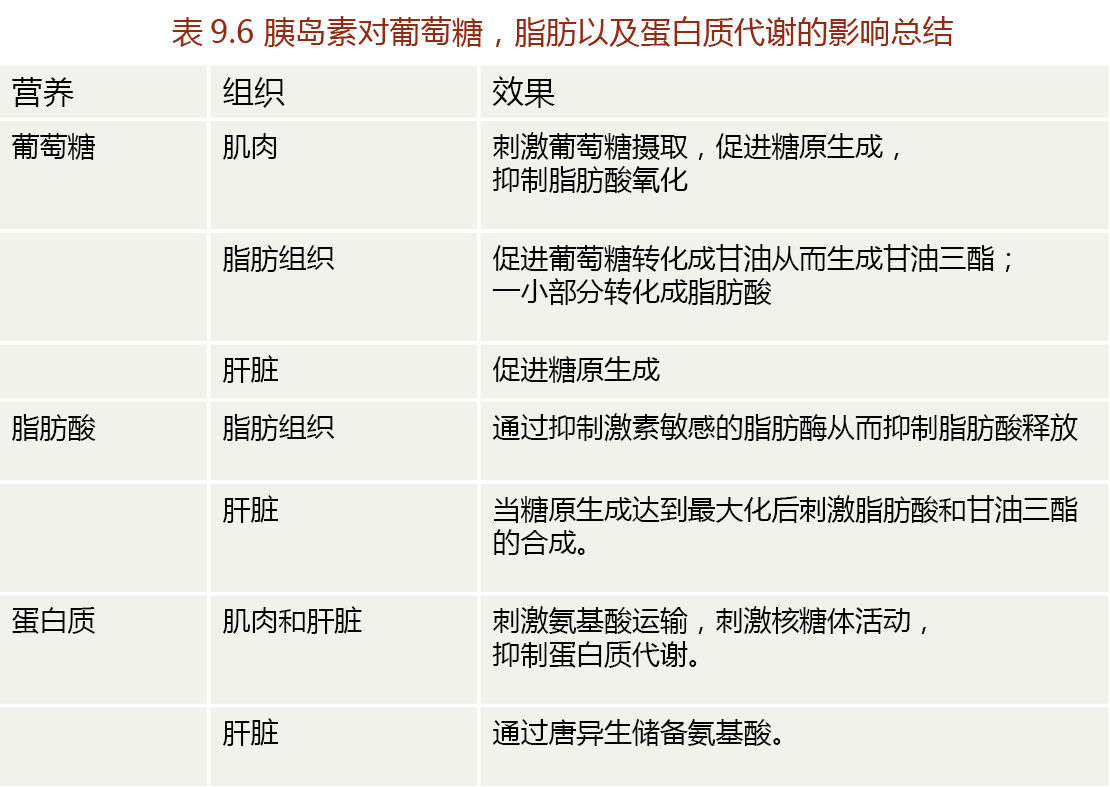
The cell membranes of liver, muscle, and adipose tissue are, for the most part, impermeable to glucose; the cell membranes of neural tissue and the kidney allow passive diffusion of glucose. Thus, glucose requires carrier proteins for passage into liver, muscle, and adipose tissue cells. A specialized carrier protein for glucose, called glucose transporter 4 (GLUT4), facilitates glucose transport only in the presence of insulin—this ensures that blood glucose remains available for the brain even when blood glucose concentrations are below the level that stimulates insulin secretion (∼120 mg/dL). The primary actions of insulin on muscle, liver, and adipose tissue are shown in TABLE 9.7.
In the presence of increased blood glucose, such as following a meal, the ß-cells of the pancreas secrete insulin (Figure 9.31). Binding of insulin to tyrosine kinase-linked receptors on the surface of cells initiates a series of signal-propagation events, involving phosphorylation, that induce glucose uptake. Binding of insulin to the cell surface side of its transmembrane receptor activates tyrosine kinase. The tyrosine kinase phosphorylates a tyrosine residue on proteins that induce GLUT4 molecules to move from the cytosol to the cell surface. Glucose can then pass through the GLUT4 membrane channels. Binding of insulin to its receptor also activates several other kinases that phosphorylate insulinrelated substrates, thus activating several glucose metabolic pathways (glycolysis and glycogenesis) as well as the expression of genes needed for glucose-related metabolism.
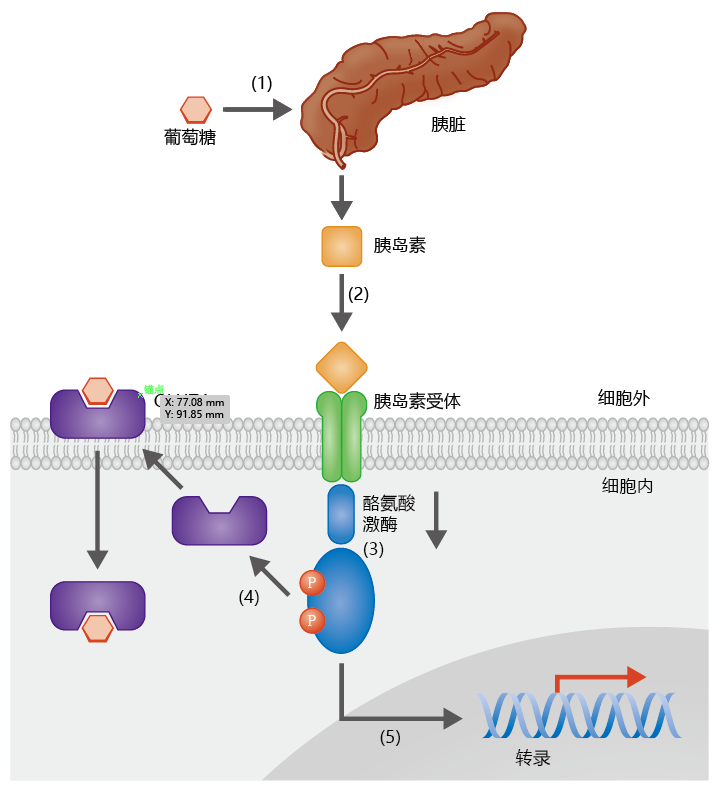
Figure 9.31 The facilitation of glucose uptake by insulin. (1) Increasing blood glucose concentration stimulates the secretion of insulin by pancreatic β-cells. (2)Insulin binds to a transmembrane tyrosine kinase-linked receptor, which (3) activates tyrosine kinase and phosphorylates protein substrates. (4) Phosphorylation of these substrates induces the movement of glucose transporter 4 (GLUT4) molecules from the cytosol to the membrane surface, allowing the uptake of glucose. (5) Tyrosine kinase also induces the phosphorylation of a number of other insulin-receptor substrates, resulting in activation of glucose metabolic pathways and gene expression.
We generally think of insulin as a hormone that regulates carbohydrate metabolism, but it also influences the metabolism of protein and fat, with the effect of sparing these energy substrates during times of glucose excess. For example, insulin inhibits the action of hormone-sensitive lipase in adipose tissue, thus preventing the breakdown of triglycerides and release of free fatty acids into the blood. This limits the amount of energy from fat that is available to other tissues and “forces” cells to use glucose for energy. In the liver, excess insulin-stimulated glucose uptake (exceeding the liver's capacity for glycogen storage) results in the accumulation of acetyl-CoA, a precursor of fatty acids and cholesterol. Thus insulin can stimulate fatty acid synthesis in the liver. The newly formed fatty acid is generally packaged as triglycerides, released from the liver into the blood, and transported to adipose tissue for storage.
Insulin can stimulate the synthesis of proteins in various cells by several mechanisms. First, insulin stimulates the uptake of amino acids following a meal, providing cells with the amino acids needed for protein synthesis. Second, insulin can stimulate translation of mRNA by increasing ribosomal activity. Third, insulin increases the rate of transcription of DNA coding for enzymes that stimulate the storage of carbohydrates, fats, and proteins. Finally, insulin inhibits the catabolism of proteins and thus limits the quantity of amino acids that can be used for gluconeogenesis.


