10.1 MODULATING BIOLOGICAL AGING
Aging is caused by random, stochastic damage to cellular molecules that leads to altered cell function. Longevity—the evolved lenth or duration of life for a species—has arisen as a by-product of genes selected for reproductive success. The biochemical and physiological mechanisms that underlie aging and longevity are not yet fully understood. Nonetheless, many biogerontologists agree on one point: the rate of aging and alterations in longevity reflect the intracellular accumulation of damaged proteins.
In this section, we discuss why aging cannot be modulated, then look at what types of research might be needed to better understand the biological basis of aging.
衰老是无法调控的
我们活着。我们变老。我们逝去。虽然衰老是我们大多数人都想停止的事情,但简单的事实是衰老是无法改变的。为了理解为什么会这样,你必须接受我们在前几章中讨论过的生物系统的三个重要原则:(1)衰老没有进化,(2)生物有机体与无生命物体遵循相同的热力学定律,(3)热力学第二定律不断地、随机地运行。由于衰老没有进化,因此没有基因调节这一过程。因此,老化必然是一个随机事件。老化的随机性是由热力学第二定律引起的。一个有机体中会发生数以百万计的反应,每一个反应都必须符合热力学第一定律和第二定律。这是宇宙中无法抗拒的事实。宇宙的力量推动每一个化学反应,甚至是最微小的化学反应,朝着增加熵和无序的方向发展。在某个时刻,在每一个有机体中,这些反应将试图在熵超过自由能的系统中发生,进而导致分子保真度的损失和受损蛋白质的积累。这将引发连锁反应,最终导致细胞功能丧失。随着时间的推移,人体内的所有细胞都会经历分子保真度的丧失。人类无法,也很可能永远无法改变这个宇宙的基本事实。
One can argue, however, that the laws of thermodynamics apply to a closed system, a system having no input from the environment. Organisms are open systems, constantly interacting with the environment, and perhaps we can intervene to offset the effect of the second law. Some suggest that, in the twentieth century, human intervention that led to the unprecedented increase in life expectancy proves that biological systems can successfully combat the second law. A close inspection of the gains in life expectancy over the past 100 years, however, suggests only that we made headway against disease. Aging remains. As shown in Figure 10.1, there was indeed a rapid increase in life expectancy during the twentieth century and the first decade of the twenty-first century. But, the increased life expectancy between 1900 and 1950 was achieved by curtailing the infant mortality rate, reducing deaths due to childhood diseases, and reducing deaths due to infections that killed most people before the end of their reproductive period. The slower gains after 1960 were the result of advances in medical technology that reduced deaths from diseases that had been major killers of older people before they reached an advanced age. For example, individuals who would have died in their fifties or sixties from a heart attack are now living into their seventies and eighties because of technologies that diagnose and fix the problems before they become fatal. Thus, the increase in life span during the twentieth century was the result of modulating age-related disease, not aging.
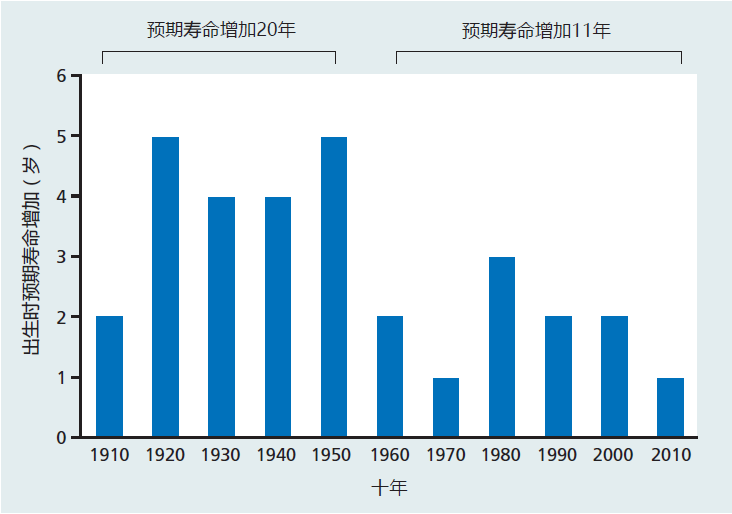
Figure 10.1 Gain in life expectancy at birth by decade, 1910–2010. Note that 60% of the gain in life expectancy occurred during the first 50 years of the twentieth century, reflecting a decrease in infant mortality and improvements in disease control. (Data from Bell FC, Miller ML. 2005. Life Tables for the United States Social Security Area 1900–2100, p. 194. Washington, DC: Social Security Administration.)
Some scientists suggest that the rapid advancements in biotechnology will lead to antiaging therapies. These researchers point to advances in stem cell research that show great promise for restoring age-related functional loss. Others suggest that organs grown in vitro from our own cells will replace those damaged by age or disease. Both types of therapy will undoubtedly be realities in the future and will lead to increased life expectancy. But the question before us is, will these interventions modulate aging? The answer is an unequivocal “no”; they will only postpone the inevitable. As soon as you fix one tissue or organ, another system will fail, and so on. Knee replacement, a common surgery in the elderly population, has not stopped kidney cells from accumulating damage. Perpetual motion in humans is impossible, just as the nineteenth-century physicists who developed the laws of thermodynamics predicted.
Mechanisms that lead to loss of molecular fidelity may be modulated in future
Since its beginning, biogerontological research has focused almost exclusively on trying to alter the process of aging by correcting damage that has already been done. This approach to modulating aging will never be successful: increasing entropy is a fundamental law of the universe, and all matter strives to reach energy equilibrium. We can, however, modulate the rate of aging by evaluating mechanisms that lead to the loss of molecular fidelity.
All age-related disease will be cured someday, and we will be left with nothing more than increasing entropy as the cause of death. If we are to modify the rate of aging, biogerontological research must focus on the mechanisms of aging, not on the mechanisms of age-related disease. Biogerontology should be evaluating why, given the laws of thermodynamics, organisms survive at all. In other words, biogerontologists need to stop asking “Why do we die?” and begin asking “Why do we live?”
It is not easy to predict what specific research will be undertaken when biogerontologists frame their research in the why-do-we-live context. With the rapid pace of discoveries in biology, any predictions made today will probably be outdated tomorrow. There are, however, some general areas of research that should be given greater focus if we are to modulate the rate of aging. These areas include genetics and gene regulatory systems.
Because evolution has selected all of our genes to ensure survival to reproductive age, it would seem appropriate to focus greater attention on genes and gene regulatory systems that maintain usable energy—that is, those genes that maintain molecular fidelity and cellular order. In general, these are the genes that regulate the repair of DNA and proteins and the removal of damaged cellular components.
The focus on genes that maintain molecular fidelity and cellular order will lead naturally to research that evaluates when systems, and what systems, are likely to be the most susceptible to the second law. Evolutionary theory provides the answer to the when: systems involved in repair and maintenance become susceptible to the second law after the organism reaches reproductive age. Thus, there needs to be a significant shift in the model generally used in aging research. Biogerontologists should begin investigations aimed at young, prereproductively active and reproductively active populations, without making comparisons with the aged (postreproductive) population. Comparison with the aged population should occur only after achieving a clear understanding of genetic pathways or regulatory systems in younger populations that are most susceptible to the second law. Then, research can begin to test whether or not these systems affect the rate of aging.
Given the current state of biotechnology, answering the question of what system or systems are most susceptible to increasing entropy is made difficult by the randomness of the second law. Biogerontologists need significant advances in genomic research (a topic discussed in Chapters 2 and 5) before they can identify genetic pathways that are likely to be important to the rate of aging (some of this research has begun in invertebrates). This will undoubtedly require a greater use of mathematical models—a method almost nonexistent in biogerontology—to help predict outcomes of age-related functional loss resulting from gene expression (or nonexpression) in young individuals.
Until general genomic research provides biogerontologists with the tools necessary for investigating the impact of increasing entropy on age-related functional loss, some areas of research might be helpful in the short term. General medical science and biogerontology have determined that the cardiovascular system tends to decay at a greater rate than other systems. Fatty streaks in arteries, precursors to an accumulation of damaged protein that leads to narrowing of the vessel, can be found in children as young as 6 months old. Using the cardiovascular system as a model, researchers can begin to understand how a loss in molecular fidelity begins. The development of certain types of cancer, particularly those likely to occur in younger populations, may also provide some understanding of why genetic pathways regulating damage and repair become altered.


