11.1 ACHIEVING THE PROMISE OF EXTENDED HEALTHSPAN
Greek mythology tells of the god Apollo granting Cumaean Sibyl one request in exchange for her virginity. She chose eternal life. Sibyl forgot, however, to include eternal youth in her request, and she spends eternity suffering the disabilities of old age. The mythology of ancient Greece reminds us that most people are unwilling to accept a long life without good health. Any life extension must and will come with extended youth and relatively good health into old age. We discuss in this section how interventions extending the healthspan will be implemented into the health-care system.
Healthspan combines measures of life span and disability
Healthspan reflects the number of years in the life span that are spent being free of time-dependent disability (for the purpose of this text, disability refers only to time-dependent functional loss or age-related disease occurring in the older population). Calculating the healthspan requires measures of life span and disability. The difference between the length of life and years spent free of disability provides information as to whether the healthspan is increasing or decreasing over time. A decrease in the difference between length of life and years spent free of disability indicates an increase in the healthspan. An increase in the difference would suggest a decrease in healthspan.
Accurate and reliable measures of life span and disability are necessary to determine if interventions aimed at increasing healthspan are effective. Healthspan will only be as accurate as the variables in its equation. Measuring the length of life is highly precise and is an objective measure because it is dichotomous, a variable with only two possibilities. One is either dead or alive. Conversely, disability is highly subjective, and its measurement in the older population presents significant challenges. The imprecision in measuring disability results from the lack of a generally agreed on definition for health and disability (Chapter 10); health and disability have several definitions. As a result, disability is a continuous variable, a variable with a number of values and open to considerable individual interpretation.
Disability as used in the calculation of healthspan will, in general, reflect the investigators' assumptions concerning the definition of aging and the population being studied. Some equate morbidity, the presence of disease, with disability during old age. You have learned, however, that modern treatments for chronic disease either prevent or significantly reduce disease-related disability. Morbidity can be a poor measure of disability in the older population. Physical functionality, how well one performs a physical task, can improve the measurement of disability. For example, activities of daily living (ADLs) (Figure 11.1) measures an individual's ability to perform basic functions needed for independent living. These six measures are useful in a clinical setting to determine if an individual with a preexisting condition can continue to take care of himself or herself without assistance. The ADLs are less useful as a measure of disability in an older population experiencing only typical time-dependent functional loss.
Physical functionality as a measure of disability in independently living individuals will often be assessed with sets of higher functioning measures than that of the ADLs. Walking speed, grip strength, lifting weights, climbing stairs, etc., have been correlated to the progression of disability in the older population. Some have criticized the use of only physical functionality as a measure of disability because it reflects the end result of a process started in the cell many years previously. It has been suggested that measuring disability in the older individual would be more precise if measures reflecting a cellular mechanism of aging were added to physical functionality. Several biological measures of immunity, endocrine function, cardiovascular health, and muscle metabolism have found wide usage as measures of health and disability in older individuals and populations. Nonetheless, no single measure or set of measures of physical functionality or biological function have reached the criteria for a true biomarker of aging (Chapter 2). Until a true biomarker of aging has been validated, measurement of disability resulting from time-dependent functional loss will remain imprecise and open to debate.
Preventing or curing chronic disease will not continue to reduce disability
You have learned throughout this text that the increased life span and the reduction in disability seen during the twentieth century were achieved by curing and treating disease. Many believe that this strategy for increasing life span and healthspan may have reached its peak. For example, between 1900 and 1950, a period focusing on preventing and curing infectious diseases in the young, the rate of life expectancy at birth rose 1 year for every 2–3 calendar years (Figure 11.2A). The period of time generally accepted as focusing on treating chronic disease in the older population, between 1950 and 2000, experienced a decline in the rate of life expectancy gain to 1 year of additional life for every 3–4 calendar years. The current rate of gain in life expectancy stands at about 1 year every 6 calendar years of life (Figure 11.2A).
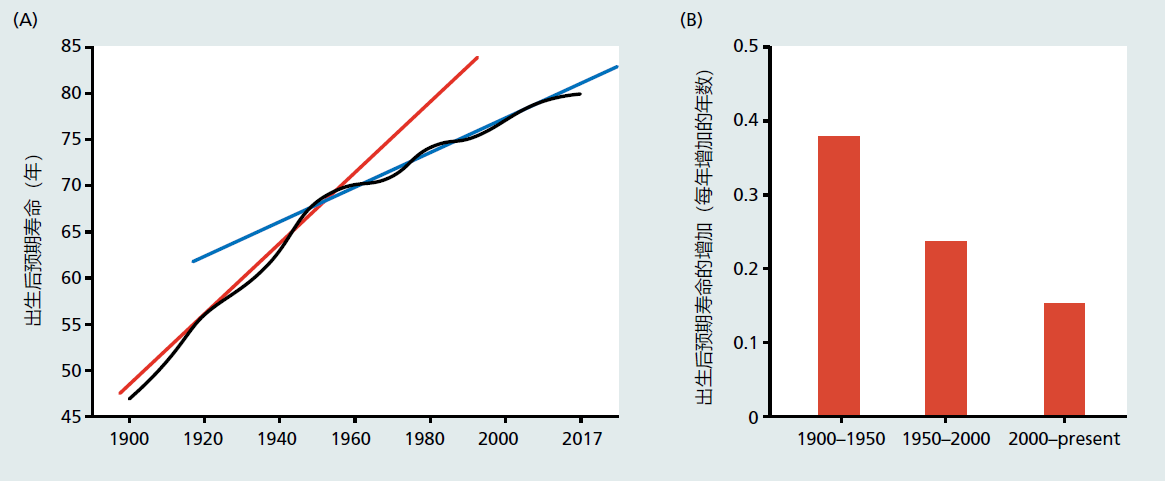
Figure 11.2 (A) Life expectancy from birth and (B) gains in life expectancy from birth in a given time period in the United States. The red and blue lines shown in (A) are the linear rates of gain for life expectancy (regression slopes) for the periods 1900–1950 (red) and 1950–2017 (blue). The linear rate of gains in life expectancy from birth significantly slowed after 1950. It was during this time that death due to infectious disease began to disappear from the top 10 causes of death. The infectious disease tuberculosis appeared for the last time in 1952 at #9. Note the flattening of the life expectance from birth in (A) starting at about the year 2014. Life expectancy at birth has remained constant since 2014 (2014 = 78.8; 2015 = 78.7; 2016 = 78.6; 2017 = 78.6).
The most current data suggest that improvements in disability from treating chronic disease have, most likely, also reached their peak. The Global Burden of Diseases, a large epidemiological study that includes data from 195 countries, reported that disability from all causes decreased by 2.3% between 2000 and 2016. Death rates in the same cohort decreased by 3.1%. That is, declines in disability did not keep pace with life extension. This means that people are living longer with disability. Several studies done in North America and Europe have shown that the proportion of people reporting limitation in one or more activities, ADLs, and other high-functioning tests, have not changed since the year 2000. The current data strongly suggest that treating chronic disease as a primary method for reducing disability is ill advised.
It has become clear that an exclusive focus on treating or curing chronic disease cannot be expected to significantly decrease disability in the older population. A new approach will be needed to reduce time-dependent disability and increase healthspan. This new direction in research will be to evaluate interventions that postpone the effects in the mechanism of aging. This conclusion arises from the fact that 95% of all chronic disease and disability occur after the age of 60. That is, the passage of time and the physical laws of the universe are the underlying cause of chronic disease and physiological decline. Postpone the effects of time on the human body, that is, delay the effects in the mechanisms of aging, and we postpone the development of chronic disease and time-dependent physiological decline. Postpone the development of chronic disease and time-dependent physiological decline, and we increase healthspan.
Improving healthspan by increasing levels of exercise and reducing caloric intake will be challenging
There exists overwhelming evidence that increasing the levels of exercise and maintaining a low-calorie diet throughout adult life postpones the effects in the mechanisms of aging and extends healthspan (Figure 11.3 and Chapter 10, Figure 10.7). However, participation by the general population in these two healthspan-increasing treatments remains low and declines with age (Figure 11.4). Data published in 2017 show that only 21% of Americans achieve the recommended levels of exercise per week. Exercise participation rates in other economically developed countries are similar. Adherence to caloric intake recommendations is also low. Recommendations for caloric intake are 2200–2500 kcals in the United States and 1600–1800 kcals for the European Union. Studies have shown that between 10% and 30% of the population in these countries meets this recommendation. The average daily intake of kcals in the United States for both men and women lies between 2800 and 3600 kcals. The EU's consumption of calories appears to be about 15%–20% lower than that of the United States but remains well above recommended values. It is, therefore, no wonder that 20%–35% of individuals in many economically developed countries are obese (about 10% of the population was obese in 1950).
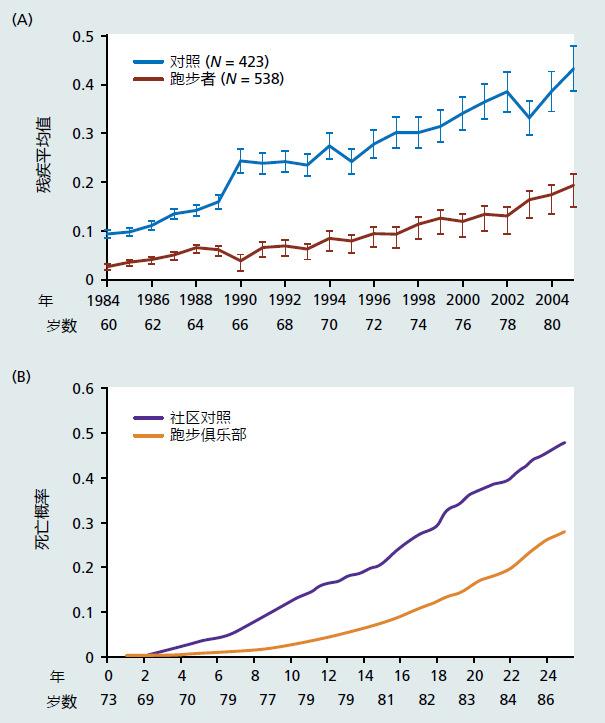
Figure 11.3 Longitudinal study of disability (A) and mortality (B) in runners (n = 423) and nonrunners (n = 538) followed for 20 years. Disability was evaluated using a questionnaire that included measurements from eight areas; rising, dressing and grooming, hygiene, eating, walking, reach, grip, and activities. Each area was scored from 0 (no difficulty) to 3 (unable to perform). The values on the x-axis in graph A represent the average score for each area. Statistical analysis showed that disability in the runners versus nonrunners was postponed by 16 years. The average age at death was postponed by 10 years. (From Fries JF. 2012. Curr Gerontol Geriatr Res. doi: 10.1155/2012/420637. A, Figure 1; B, Figure 3. With permission.)
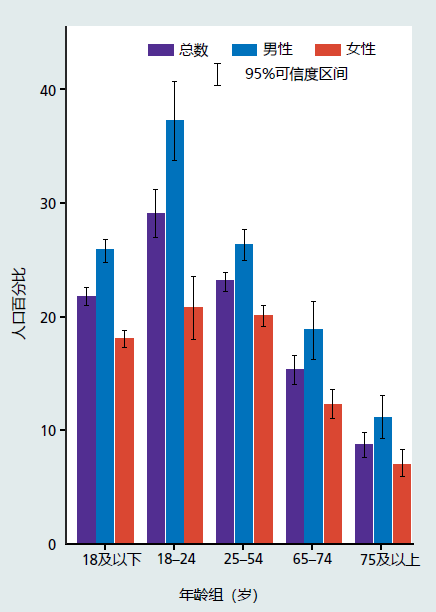
Figure 11.4 Percent of population that meets the weekly amount of aerobic and strength training exercise recommended by the National Institutes of Health. (From Clarke TC et al. Early Release of Selected Estimates Based on Data from 2016 National Health Interview Survey. National Center for Health Statistics. May 2017. http://www.cdc.gov/nchs/nhis.htm, Figure 7.5.)
Reluctance of the general population to adopt increases in exercise and reduce caloric intake reflects many issues. And, of course, not all problems and issues can be addressed simultaneously. Most economically developed countries have chosen to focus their attention on finding ways to make exercise programs and caloric reduction protocols prescribable treatments (see later discussion). This approach was chosen because it addresses many problems that form barriers to adapting exercise and calorie reduction programs to improve health. For example, current recommendations for exercise (Figure 11.5) and diet (Figure 11.6) offer only general advice and lack specifics. These recommendations are often outdated, and the vast majority of the population are unaware that guidelines exist. (The exercise guideline presented here was developed in 2008.) Most people get their information on exercise and diet from the Internet, casual discussions with friends and family, articles and books in the lay press, or the well-meaning but insufficiently trained health club worker. The lack of scientifically accurate and specific exercise and diet protocols can lead to frustration on the part of many individuals. This frustration can inhibit individuals from initiating and maintaining exercise and diet modification.
Economic considerations also present a significant deterrent to exercise and diet modification. Individuals wishing to undertake these treatments may find the cost prohibiting. Many types of exercise, strength training, and yoga, for example, require membership to health clubs or other private businesses. In addition, many parts of the world have climates that prevent exercise outdoors during a significant portion of the year. Exercise equipment, treadmills, stationary bikes, etc., are expensive. Those needing diet counseling aimed at reducing calorie intake will also find that services provided by a licensed professional are expensive and rarely covered by insurance. Medicare, for example, will cover the cost of nutrition therapy services only if the patient has diabetes or kidney disease.
Prescribable protocols will help to increase participation in exercise and diet treatments
Most economically developed nations have begun the process that will eventually lead to prescribable protocols for exercise and diet. For example, the National Institutes of Health (NIH), the agency that sets the health agenda for the United States, has embarked on an ambitious research program to determine the underlying molecular events that lead to the health benefits of exercise (see “The Road Ahead” in Chapter 10). This program provides funding for researchers to use modern biotechnologies, genomics, systems biology, etc., that will ultimately lead to the creation of exercise protocols specific to the health needs of a single individual. Thus, precise, scientifically accurate, and individualized information on which type and how much exercise to perform will be given to the patient, in prescription form, by a physician or other highly trained health-care professional. The frustration and subsequent deterrent to physical activity that arise from lack of credible information will, in large part, be eliminated.
The decision to place the dissemination of specific exercise information into the hands of the health-care professional was a deliberate strategy to provide the physician with the power of prescription. A physician's authority to prescribe implies that the therapies have been approved by an oversight government agency, the U.S. Food and Drug Administration (FDA), for example, as safe and effective. Once the safety and effectiveness of a specific exercise protocol has been certified through rigorous scientific review, a review process similar to that for pharmaceuticals, governments and insurance companies have the legal protection they need to cover costs associated with physical activity. This may include, for example, health club memberships, appropriate running shoes, heart rate monitors, etc. (some health insurance companies and HMOs in the United States have already begun to reimburse for health club memberships). Through this or a similar strategy, the economic constraints to initiating and maintaining an exercise routine can be reduced.
Medical interventions postponing the proximal mechanisms of aging are being developed
Although no questions remain that exercise and caloric reduction will postpone time-dependent disability, it is likely that prescription exercise and diet treatments will have only a modest effect on participation rates. The effects of exercise and diet on health have been known for decades, including their effects in the older individual. Yet, participation levels remain dismally low (Figure 11.4). Investigations evaluating exercise participation rates in cardiac rehabilitation (CR) also suggest that prescribable exercise protocols may have only a modest effect. Cardiac rehabilitation (CR) protocols are one of the very few prescribable exercise and diet treatments that exist currently. However, these programs see no more than 20%–40% adherence rates. These low rates of protocol adherence are quite surprising given that CR significantly reduces the risk of a second heart attack. Clearly, more traditional styles of treatments, pharmaceuticals, gene therapies, etc., will be needed and combined with exercise and diet intervention in order to have the greatest impact on postponing the effects in the mechanisms of aging. These medical interventions are initially focusing on the proximal mechanisms of aging.
Throughout this text, we have described many proximal mechanisms of aging, including oxidative damage, shortened telomeres, and the dysregulation of genetic pathways, to name a few. You learned that substantial evidence exists in laboratory animals to suggest that interventions targeting individual proximal mechanisms of aging postpone disability and extend healthspan and life span. This has led to great optimism that development of pharmaceuticals or other types of traditional intervention aimed at postponing disability in the older population is now within reach. We agree; however, it is likely that introduction of such interventions remains decades away. The reason for this lies with the complex nature of aging itself.
We have emphasized that aging is a random or stochastic process caused by a loss in molecular fidelity. The randomness arises from an almost infinite number of variables that, throughout a lifetime, can affect molecular fidelity. As such, every individual or small group of genetically similar individuals has a unique set of variables that determine the rate of aging. You also learned that no single mechanism of aging determines a person's rate of aging. Together, the high degree of randomness and the multiple mechanisms of aging present researchers with significant challenges in the quest for an intervention aimed at postponing time-dependent disability. A single intervention may be effective at postponing one mechanism of aging and within a single person. However, it is highly likely that this same intervention may have little effect in postponing disability in another individual. This means that the most effective interventions will be ones that work in the majority of the population. Moreover, one mechanism at a time will be as ineffective as treating separate chronic diseases has been at increasing life span (see previous discussion). Postponing the effects in the proximal mechanisms of aging will require that the treatment be highly individualized and capable of affecting multiple mechanisms of aging simultaneously.
Individualized medications affecting multiple proximal mechanisms of aging will be introduced after quantitative methods associated with precision medicine replace the observational science of disease-centered medicine (see Chapter 1, section titled, “Reductionism Can Predict Emergent Properties in Simple Biological Systems; Complex Systems Require Quantitative Methods”). However, precision medicine is in its infancy, and many of the methods needed for development of individualized medications aimed at the proximal mechanisms of aging have yet to be elucidated (see Chapter 1, section titled, “How Biogerontologists Study Aging: Systems Biology”). As such, providing even the most liberal timeframe in which medication affecting multiple proximal mechanisms of aging will be introduced remains imprecise. Nonetheless, the discipline of biogerontology has, in general, begun its transition into quantitative biology. The National Institute of Aging (NIA) is part of the overall NIH effort to provide funding for research associated with precision medicine. Many laboratories that were once focused exclusively on reductive science are building the necessary collaborations with systems biology experts, mathematicians, and engineers that are necessary to be successful at quantitative biology within biogerontology. We can now only wait to see how quickly methods of quantitative biology influence discovery of medications that delay the effects in the proximal mechanisms of aging and postpone disability.


