9.4 与衰老相关联的心血管系统疾病:心血管疾病
Like other physiological systems discussed in this chapter and in Chapter 8, the cardiovascular system undergoes slight to moderate functional loss with age. In most cases, the functional loss does not impede daily activities, but it can reduce a person's ability to respond to stress or overload. As we discussed earlier in the chapter for neural pathologies, an important question to ask in relation to aging and the cardiovascular system is not so much what changes occur but why these changes progress to disease in some individuals but not in others. And, as for the neural pathologies, biogerontologists have yet to answer that question.
In this section, we explore three common age-related diseases of the cardiovascular system: coronary artery disease (atherosclerosis), stroke (cerebrovascular incident), and high blood pressure (hypertension). We begin with a very brief discussion of environmental factors that affect the cardiovascular system.
9.4.1 环境因子 influence age-related decline in the cardiovascular system
Defining nondisease, age-related alterations in the cardiovascular system has proved difficult. This problem arises because environmental factors such as diet, exercise, and smoking have significant effects on the rate of aging of the cardiovascular system. For example, the elasticity and compliance of the aorta and other large arteries decrease with age, often resulting in an increase in systolic blood pressure and hypertension. However, several studies have shown that physically active, aged individuals have significantly lower systolic blood pressure than age-matched sedentary people. Smoking exacerbates the loss of distensibility of the aorta and increases systolic blood pressure to a greater degree than is observed in nonsmoking, sedentary older individuals.
The physiological response of the heart to an increased demand for systemic flow does change with age and might not be influenced by environmental factors. Recall that the heart regulates its output based on the demands of the tissues for oxygen. During the initial demand for greater blood flow, such as during exercise, cardiac output increases as a result of an increased heart rate, while stroke volume remains the same. The increased heart rate is caused by stimulation of the sinus node by neurohormonal factors, which induces an increased rate of action potential generation. With age, the number of adrenoreceptors (receptors that bind epinephrine and norepinephrine) in the sinus node seems to decrease. This results in two age-related alterations in heart function. First, the increase in heart rate during the initial stage of increased metabolic demand occurs more slowly as we grow older. Thus, it takes longer for older individuals to adjust to increased metabolic demand. Second, the decrease in adrenoreceptors with aging results in a decrease in the maximal heart rate that can be achieved. This means that total cardiac output during high levels of exercise declines—that is, the maximum amount of work performed during exercise declines with age. The heart can compensate for the age-related decline in maximal heart rate by increasing stroke volume, although, because of the Frank-Starling mechanism, the absolute quantity of stroke volume has its limits. This compensation does not, however, offset the loss in total cardiac output caused by the decline in maximal heart rate.
Age-related changes in arteries and veins are also influenced by environmental factors. Recall the discussion on age-related changes in skin in Chapter 8. You learned that many of these changes result from increased cross-linking of collagen fibers. Cross-linking decreases the elasticity of connective tissue, resulting in more rigid structures. Now, look at Figure 9.16 and notice that arteries and veins have a substantial amount of connective tissue, containing mostly collagen. Moreover, the internal and external elastic membranes are composed of elastin, which can also be heavily cross-linked, with a similar decrease in distensibility. Since arteries help to regulate blood flow and pressure in direct proportion to their ability to expand and contract, a decrease in distensibility will affect blood flow and pressure. While a slight loss in vessels' ability to expand and contract is widely accepted as a normal age-related event, a lack of physical activity has been shown to exacerbate the loss. Smoking and poor diet can also have a significant effect on the functioning of blood vessels.
9.4.2 Arterial plaques can lead to atherosclerosis and ischemic events
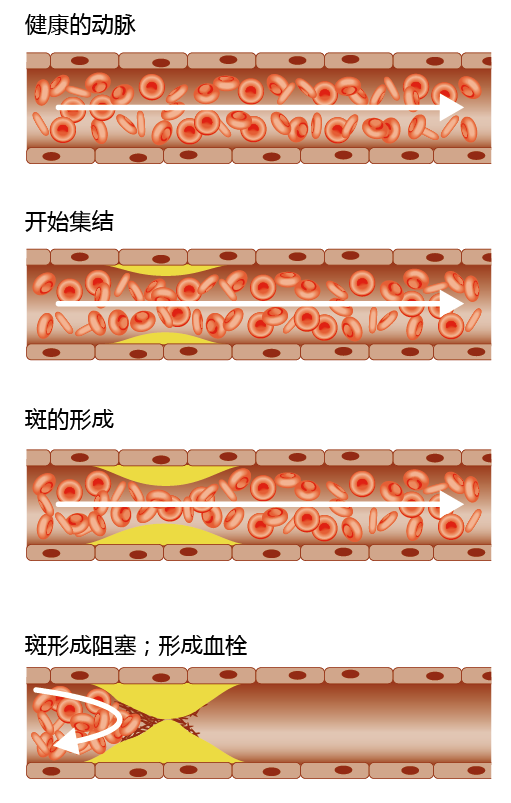
Most individuals over the age of 60 have some buildup of arterial plaques, fatty deposits inside arterial walls. As autopsies have shown, the formation of plaques begins as fatty streaks in the major arteries of the heart as early as six months of age. For the majority of individuals, arterial plaques do not become large enough to disrupt blood flow. However, for reasons still to be determined, arterial plaques can progress to atherosclerosis and disrupt blood flow. If the atherosclerosis is severe enough, blood flow can be blocked, resulting in decreased oxygen delivery to tissues, a condition known as ischemia (Figure 9.19). This can lead to the death of the tissue.
Figure 9.19 Stages of atherosclerotic blockage. The formation of plaques begins as fatty streaks in the major arteries. As plaques form, they cause an occlusion at the primary site of the lesion. To cause an ischemic event, the plaque must cause at least an 85% occlusion. Occlusion of a vessel that results in an ischemic incident usually results from rupture of the plaque and release of a thrombus.
An atherosclerotic lesion typically forms just downstream of an arterial branch point and can block an artery by two means: (1) a thrombus at the primary site (also known as the focal point) of the lesion, or (2) an embolus, a piece of the thrombus that has broken loose, block-ing a narrower part of the artery downstream from the original lesion (Figure 9.20). Not all atherosclerotic lesions result in ischemia, however. For example, autopsies have shown that some lesions rupture and release an embolus too small to induce an ischemic event. The plaque is repaired and may rupture again, releasing a larger embolus. In some cases, the plaque causes the affected artery to “grow” a new vessel around the occluded area, resulting in a clinically silent lesion.
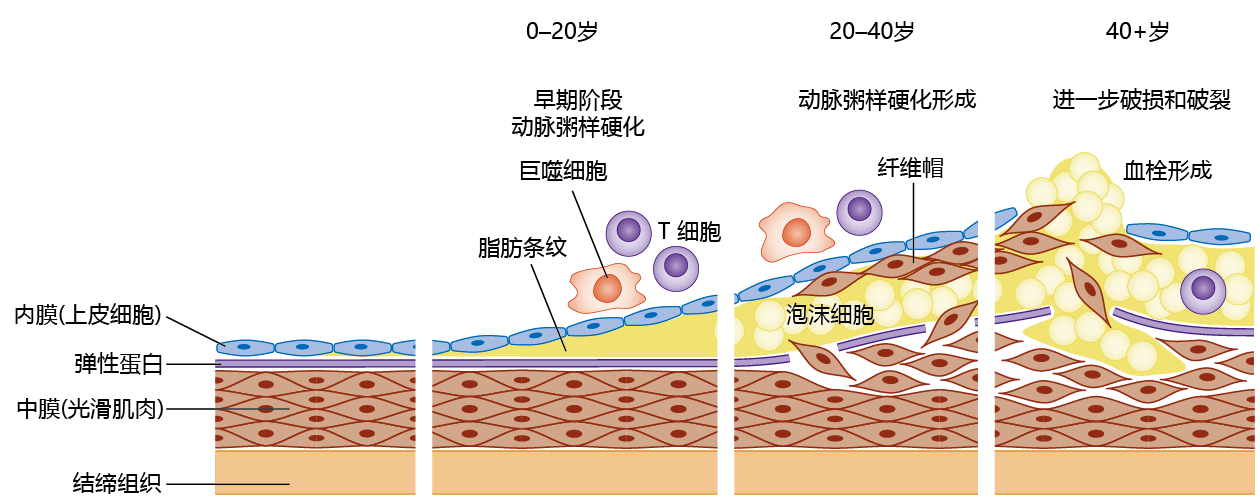
Figure 9.20 Pathogenesis of atherosclerosis over the life span. In the early stages of pathogenesis, observed in individuals as young as six months of age, the tunica intima shows an accumulation of fatty substances, appearing as fatty streaks, under the endothelial cells. The immune system recognizes the wall thickening as an antigen and directs T cells and macrophages to the site. With attack by the immune cells, the elastic lamellae between the tunica intima and media rupture, and smooth muscle cells invade the media to create a fibrous cap over the highly thrombotic foam cell component. The swelling of the artery wall is called an atheroma.
The formation of an atherosclerotic plaque large enough to cause an ischemic event takes 40– 50 years in humans. The fatty streaks in arterial walls are composed primarily of cholesterol in the form of oxidized low-density lipo蛋白质 (LDLs). The invasion of LDLs into the tunica intima layer of the vessel damages the endothelial cells, and this initiates an inflammatory response. The mixture of immune cells, mostly T cells and macrophages, and cholesterol deposits forms new structures known as foam cells. The foam cells weaken the arterial wall, allowing smooth muscle cells to invade the fatty plaque and form a fibrous cap over the foam cells (something like a scab forming over a wound on your skin). As the plaque grows during a 20- to 30-year period, it calcifies and forms its own matrix, consisting primarily of collagen. That is, the atherosclerotic plaque becomes highly thrombotic. The reason for rupture of a fibrous cap and release of the embolus remains largely unknown, but the rupture mostly involves a weakening of the area due to repeated exposure to macrophages, as well as cell death of the smooth muscle.
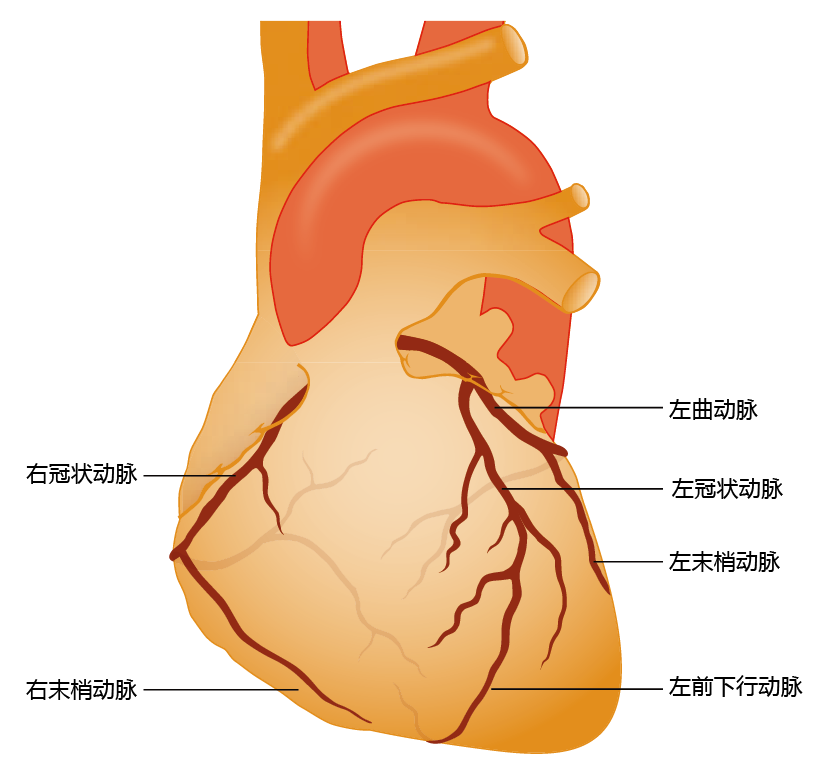
Ischemic heart disease occurs when a large artery of the heart, one of four coronary arteries (Figure 9.21), becomes blocked by an atherosclerotic lesion, and the blood flow to heart tissue decreases. If the blockage reduces blood flow by more than 85% of normal, the lack of oxygen causes cardiac tissue death; one would experience this as a heart attack, or myocardial infarction. In turn, the normal electrical conductivity of the heart becomes disrupted and the heart fails to contract, causing a significant drop in cardiac output and damage to other areas of the body. If too much cardiac tissue is affected by the blockage in blood flow, the beating rhythm of the heart is disrupted, leading to fibrillation, the uncontrolled and dis-synchronous beating of the heart. Atrial or ventricular fibrillation causes a complete loss of cardiac output and death.
Figure 9.21 The coronary arteries. Note that the coronary arteries descend directly from the aorta and thus carry highly oxygenated blood. A blockage in one of these arteries can lead to a myocardial infarction (death of tissue due to lack of oxygen), leading to tissue death and decreased cardiac output.
The mechanism leading to a stroke, or cerebrovascular incident, is similar to that causing ischemic heart disease, except that the ischemic event occurs in the brain. In most cases, the blockage resulting in an infarction is caused by an embolus that has broken off from an atherosclerotic lesion in the carotid arteries. However, some strokes can arise from the rupture of an internal brain artery and subsequent hemorrhage. The type of loss in systemic function resulting from a stroke depends on the area of the brain affected. Strokes can affect any part of the brain, but they are typically described in terms of injury to the right or left hemisphere. Right-hemisphere stroke affects motor function on the left side of the body, often causing some paralysis to the arm and leg; it also causes difficulty with judging distance and other spatial relationships, as well as impairment in the ability to understand how parts are connected to the whole. As one would expect, left-hemisphere stroke causes loss of motor control on the right side of the body. Righthemisphere stroke survivors also have a wide range of speech and language problems and sometimes develop slow and cautious behavior. Strokes can also occur in the cerebellum and brain stem. Since the cerebellum coordinates movement, strokes in this area affect balance, coordination, and reflexes. The brain stem controls most of our autonomic functions, including breathing, blood pressure, and heart rate, and strokes in the brain stem are often fatal.
9.4.3 Risk factors for atherosclerosis are a mixture of 遗传和环境条件
The mechanism underlying the progression of arterial plaques to the disease atherosclerosis remains to be determined. Four factors are considered major risks for the development of atherosclerosis: age, smoking, high serum cholesterol levels (hyperlipidemia), and high blood pressure (hypertension). These four are known as primary risk factors, because they can independently lead to atherosclerosis. Other factors such as obesity, lack of physical activity, and psychological stress are known as secondary risk factors; these must occur in association with one of the four primary risk factors in order to promote the development of atherosclerosis. Risk factors for atherosclerosis are also often classified as controllable (environmentally based) or noncontrollable (genetically based). Smoking, obesity, lack of physical activity, and psychological stress are clearly controllable risk factors. Age cannot be controlled. Hyperlipidemia and hypertension are the consequence of environmental insults to individuals who are genetically susceptible.
9.4.4 Statins reduce the synthesis of cholesterol in the liver and lower serum cholesterol
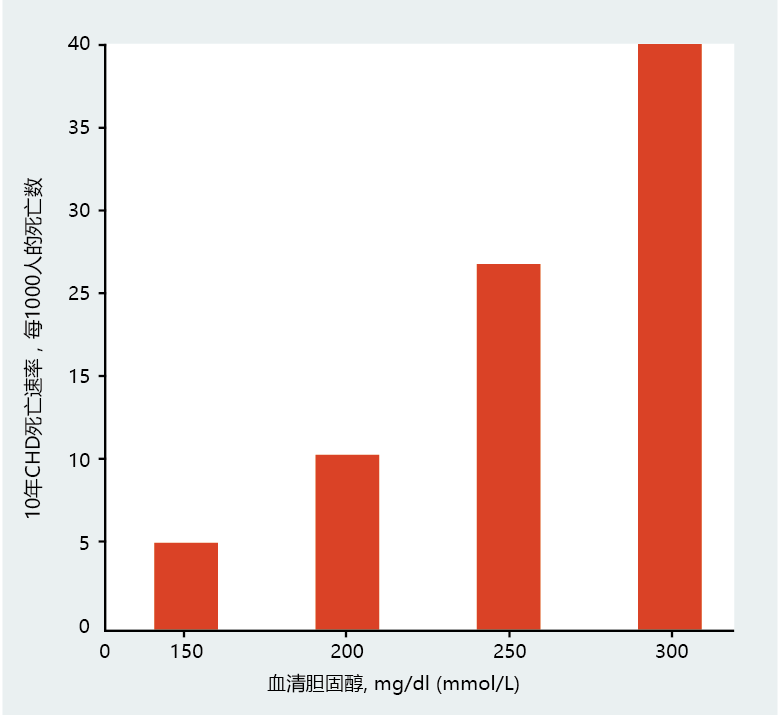
Recall that atherosclerotic plaques begin as fatty streaks in the arteries, containing primarily cholesterol. Several investigations have shown that the higher the concentration of serum cholesterol, the greater the risk of developing atherosclerosis and dying from the disease (Figure 9.22). Since food contains cholesterol, mostly in meats, reducing dietary cholesterol or total amount of dietary fat has been seen as an effective way of reducing serum cholesterol. (Remember, it is serum cholesterol, not dietary cholesterol, that is the risk factor.) Several studies during the 1970s and 1980s supported this view. Subsequent studies have refined this view, however. The results suggest that dietary management of serum cholesterol may be effective only for individuals genetically predisposed to high serum cholesterol—individuals who are known to oversynthesize LDLs, the type of cholesterol most often found in atherosclerotic plaques. The primary role of LDLs is to transport cholesterol in the blood from the liver to body cells.
Figure 9.22 Serum cholesterol and the risk of death from coronary heart disease (CHD). (Data from J.I. Cleeman. and C. Lenfant, JAMA 280:2099 –2104, 1998. With permission from the American Medical Association.)
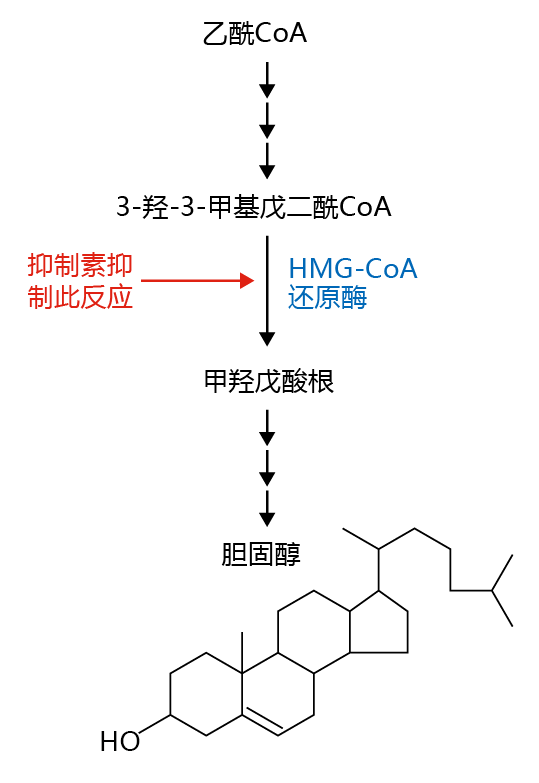
Cholesterol is a vital component of cellular membranes that provides stability to the phospholipid bilayer. While all cells can synthesize cholesterol, 70–80% of LDL synthesis occurs in the liver. Thus, for genetically predisposed individuals with high serum cholesterol, prevention of the synthesis of liver LDLs by drugs known as statins (the most commonly prescribed is Lipitor ® ) has proved effective in lowering the risk for atherosclerosis. Statins work by inhibiting an enzyme involved in the synthesis of cholesterol. In this synthetic pathway, acetyl-CoA, the end product of fatty acid breakdown (see Figure 4.19), is converted to 3-hydroxy-3-methy1glutaryl coenzyme A (HMG-CoA) in a series of reactions (Figure 9.23) . The enzyme HMG-CoA reductase catalyzes the rate-limiting step of the pathway, the reduction of HMGCoA to mevalonate, a precursor of cholesterol. Inhibiting the enzyme HMG-CoA reductase prevents cholesterol production.
Figure 9.23 Cholesterol synthesis and the action of statins in reducing cholesterol synthesis. The broken arrows indicate steps in the pathway that are not shown.
Statins have two other effects. First, reduced cholesterol synthesis in the liver up-regulates the synthesis of the liver's LDL receptors. Thus, the uptake of serum LDL by the liver increases and serum levels of LDL fall. Second, statins raise high-density lipo蛋白质 (HDL) levels by about 5%, though the reason for this is unclear. Since HDL removes cholesterol from the blood and transports it to the liver for catabolism, the lowering of total serum cholesterol by statins may involve this HDL-related mechanism.
Like all drugs, statins have side effects, but they tend to be more annoying than serious physiological problems and affect only about 5% of users. These side effects include headaches, memory problems, muscle pain or weakness, and weight gain. More serious side effects occur in less than 1% of users and vary from one individual to another.
9.4.5 Hypertension is the most common chronic condition in the aged
More than 50% of individuals 65 years and older in the United States have hypertension, which is defined as a consistent blood pressure of ≥140 mm Hg for systolic or ≥90 mm Hg for diastolic (140/90). The mechanisms underlying the development of hypertension are not known. We can conclude, however, that the increase in pressure reflects a resistance to flow (arterial stiffening), since stroke volume does not increase with age. Although the etiology of hypertension has yet to be described, several physiological and environmental factors are known to be associated with this disease. These include decreased compliance of the arteries (increased stiffness), declining adrenergic function, kidney disease, obesity, lack of exercise, and smoking. While mortality from hypertension remains low (58/100,000 in the United States), hypertension can lead to an increased risk for other forms of heart disease. For example, most studies find that a reduction of 10–15 mm Hg in systolic blood pressure can reduce the risk of an ischemic event by as much as 45%.
Treatments for hypertension include a reduction in salt intake, increased exercise, and drugs that reduce vasoconstriction and blood water volume. The effectiveness of reduced salt intake and increased exercise in reducing hypertension remains somewhat uncertain. While several studies have shown that both of these interventions can be effective in some people, there are many people who do not respond. Moreover, the reduction in blood pressure resulting from increased physical activity and/or decreased salt intake is modest, often no more than a 10% reduction in systolic and diastolic pressures. The limited ability of these interventions to significantly reduce blood pressure in the entire population suggests that hypertension has a strong genetic component.
Drug therapies have proved to be the most beneficial way of controlling blood pressure. There are several classes of drugs used to treat hypertension; the three most common are beta-blockers, angiotensinconverting enzyme (ACE) inhibitors, and diuretics. Beta-blockers block beta-adrenergic receptors. ACE inhibitors inhibit the conversion of angiotensin I to angiotensin II. Recall that both norepinephrine and angiotensin II are strong vasoconstrictors (see Table 9.3). Diuretics typically block the formation of antidiuretic hormone (vasopressin), a hormone that prevents passage of water from blood into the urine. Thus, diuretics increase water loss (in the urine) and reduce blood volume. All three types of treatment lower the resistance to blood flow and thus act in accordance with the laws of flow described earlier in the chapter. Recall that a decrease in resistance decreases pressure (see Equation 9.3).
9.4.6 Heart failure results in a decline in cardiac output
The causes of heart failure (also known as congestive heart failure) cannot be defined as clearly as the causes of ischemic-related disorders. Heart failure results from a number of conditions that range from infection of the myocardium to abnormal retention of salt and water. Nonetheless, the deterioration in the physical properties of the myocardium that leads to heart failure is the same, regardless of the underlying cause. In all cases, heart failure can be defined as the inability of the myocardium to generate the force of contraction needed to eject the blood volume required to meet the oxygen needs of the body—that is, an abnormal decrease in cardiac output.
Heart failure is classified into two types: difficulty in filling the heart (diastolic dysfunction) and difficulty in ejecting blood from the heart (systolic dysfunction). Diastolic dysfunction occurs primarily when the ventricle wall has become stiff, with a consequent decrease in compliance and end diastolic volume; the contractility of the heart often remains unaffected. That is, although the heart cells are working normally, the heart muscle cannot expand sufficiently to accommodate the volume of venous return. The declining end diastolic volume causes a decrease in the force of contraction and the amount of blood ejected during systole. In turn, peripheral tissues do not receive sufficient blood to adequately oxygenate the cells.
Systolic dysfunction results primarily from damage to heart muscle—that is, myocardial infarction. Systolic dysfunction, like diastolic dysfunction, results in decreased stroke volume, regardless of the end diastolic volume. However, the mechanism underlying the decrease in stroke volume differs from that in diastolic dysfunction. The loss of heart tissue due to an infarction reduces the total number of myocytes that participate in contraction during systole, so the contractility of the heart is reduced; compliance may remain unchanged.
The physiological response to heart failure, diastolic or systolic, is progressive and results, for the most part, in fluid retention, or edema. The mechanisms leading to edema caused by heart failure are complex, but the simple explanation is that blood in the veins backs up. The resulting increase in osmotic pressure causes fluid to leave the veins and enter the interstitial spaces. The most serious location for edema is the lungs, in a condition known as pulmonary edema. An increase in fluid in the lungs impairs gas exchange. Individuals with heart failure literally begin to drown in their own body fluids.
9.4.7 Prevalence is a better descriptor of cardiovascular disease than is mortality
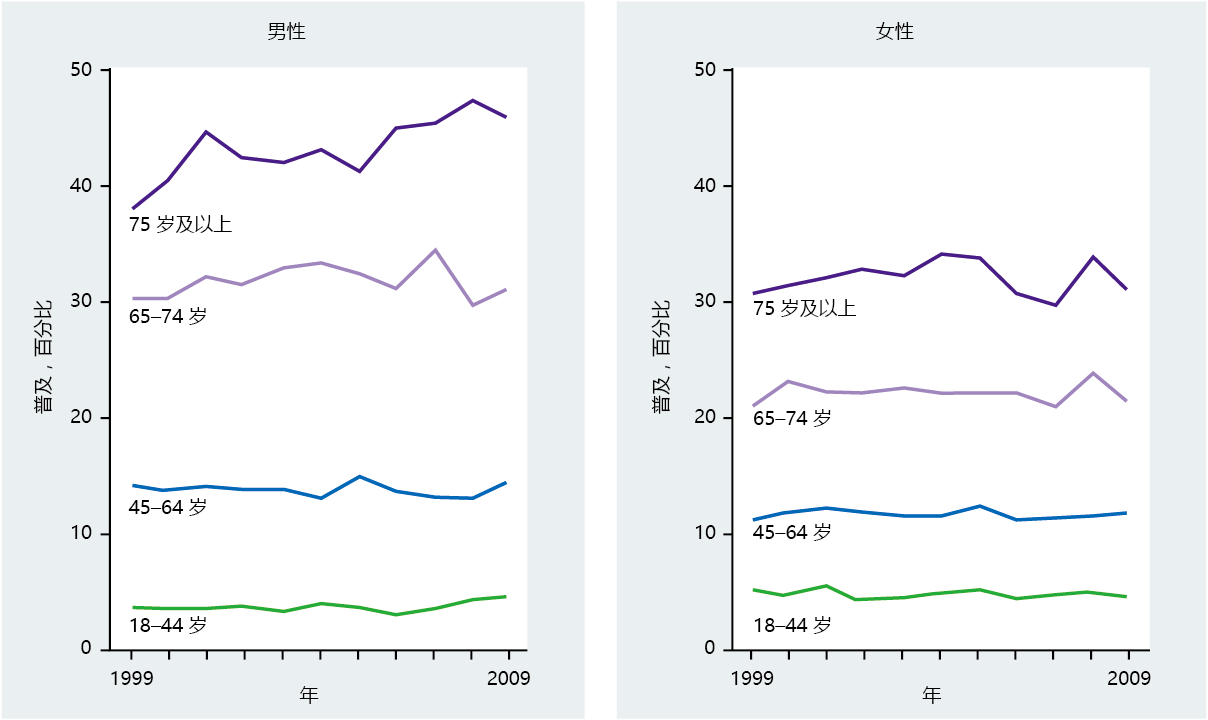
In the United States between 1950 and 2007, deaths caused by diseases of the heart in individuals over the age of 65 dropped by 41% for males and 39% for females (Figure 9.24). Nonetheless, ischemic heart disease remains the number one cause of death in older individuals in the United States and other developed countries, and the rate of mortality increases with advancing age. Mortality rate was once considered the defining measure of the age-related occurrence of cardiovascular disease, because medical science had not yet developed procedures to prevent death in patients with ischemic heart disease. Today, however, improved diagnostic techniques significantly reduce deaths due to cardiovascular disease. With the introduction of bypass surgery, angioplasty, and insertion of stents, along with a greater awareness of the impact of diet on disease prevention, cardiovascular disease is now, to a large degree, a chronic rather than a lethal condition. Thus, prevalence rather than mortality may be a better estimate by which to gauge the effect of cardiovascular disease in the aging population (Figure 9.25)
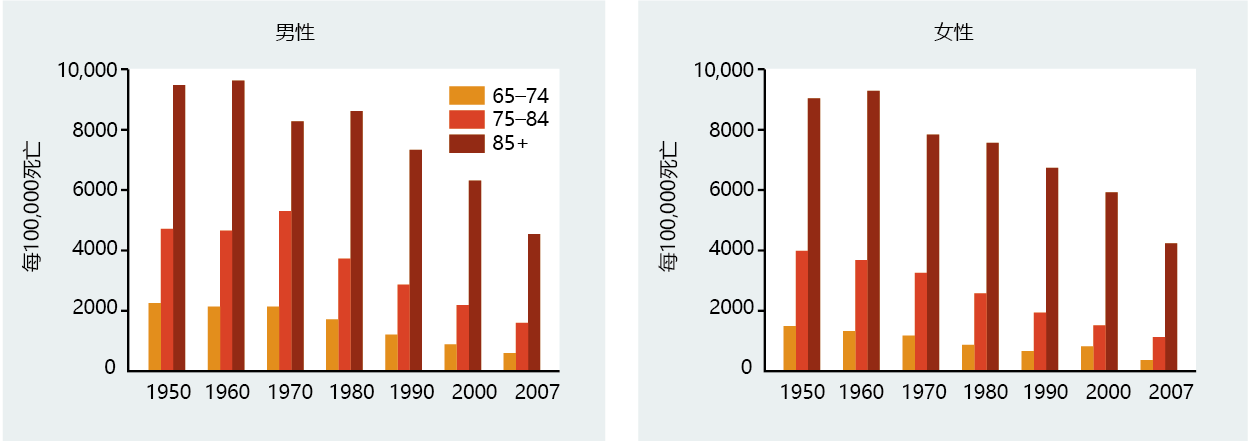
Figure 9.25 Prevalence of heart disease in men and women, United States, 1999–2009. The prevalence of heart disease has remained stable or increased slightly at the same time that death rate was dropping (see Figure 9.24). Together, decreasing death rates with increasing prevalence indicates that medical advances have extended longevity for individuals with this disorder. (Data from National Center for Health Statistics, Health, United States, 2010: With Special Feature on Death and Dying, Hyattsville, MD: Centers for Disease Control and Prevention, 2011.)