9.3 THE CARDIOVASCULAR SYSTEM
In this section, we consider a few basic physiological properties that govern blood flow and the subsequent oxygenation of tissue in the normally functioning cardiovascular system. We are taking this approach because impedance to blood flow (and thus oxygenation) is the major factor underlying age-related and/or disease-related decline in many organs and tissues.
9.3.1 The cardiovascular system is a closed system of fluid transport
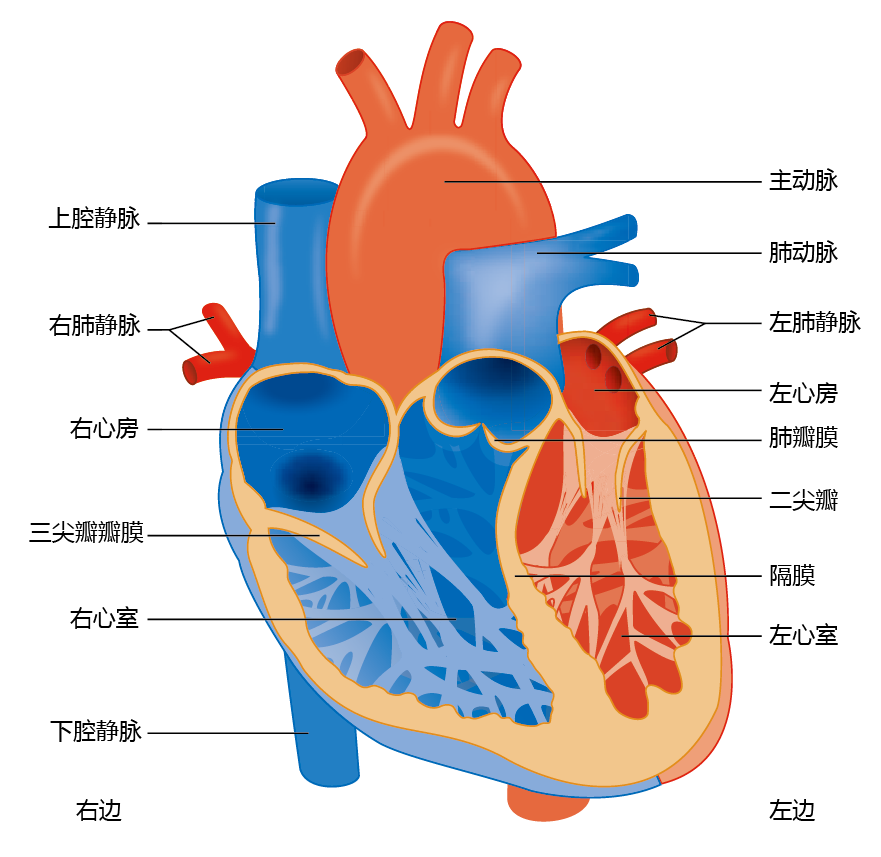
The cardiovascular system, like all closed systems of fluid transport, consists of a central pump (the heart) and conduits (arteries and veins) that carry the fluid to target structures (cells) and back to the pump. The heart consists of two separate pumps: the right side delivers oxygenpoor blood to the pulmonary system (lungs), and the left side supplies oxygen-rich blood to the body (Figure 9.14). Each side of the heart has two chambers, the atrium and the ventricle. The atrium acts as a primer or regulator of blood volume for the ventricle. The right atrium receives oxygen-poor blood from the superior vena cava, and the left atrium receives oxygen-rich blood from the pulmonary vein. The atria are separated from the larger and more powerful ventricles by one-way valves (the tricuspid valve on the right side and the mitral valve on the left side). The ventricles eject blood from the heart through the aortic valve on the left and the pulmonary valve on the right.
Figure 9.14 Anatomy of the human heart.
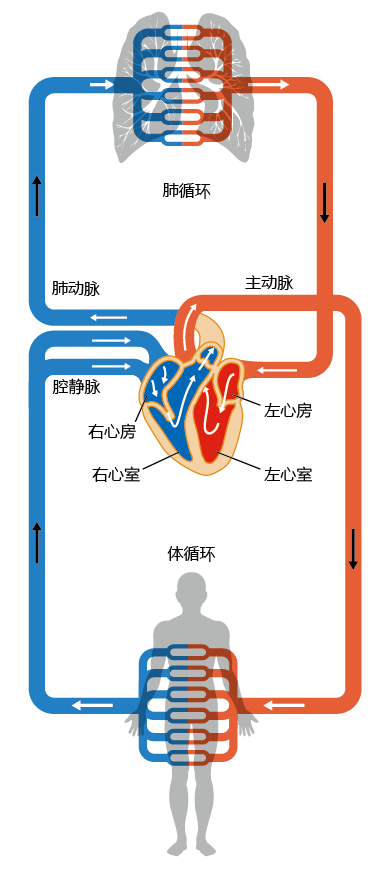
The circulatory system, like the heart, consists of two separate but connected systems, the pulmonary circulation and the systemic circulation (Figure 9.15). Vessels leaving the heart are called arteries and vessels entering the heart are called veins. The circulatory system is organized so that, in the direction of blood flow, arteries become progressively smaller and veins become progressively bigger. This arrangement has evolved so that the exchange of gases between the red blood cells and tissue cells occurs efficiently. The lumen of the capillaries (the smallest vessels), where gas exchange occurs through the semi-permeable walls, has a diameter equal to that of one red blood cell, resulting in a very low ratio of dead space to surface area.
Figure 9.15 The systemic and pulmonary circulations. Oxygenated blood is represented by red ; deoxygenated blood, by blue.
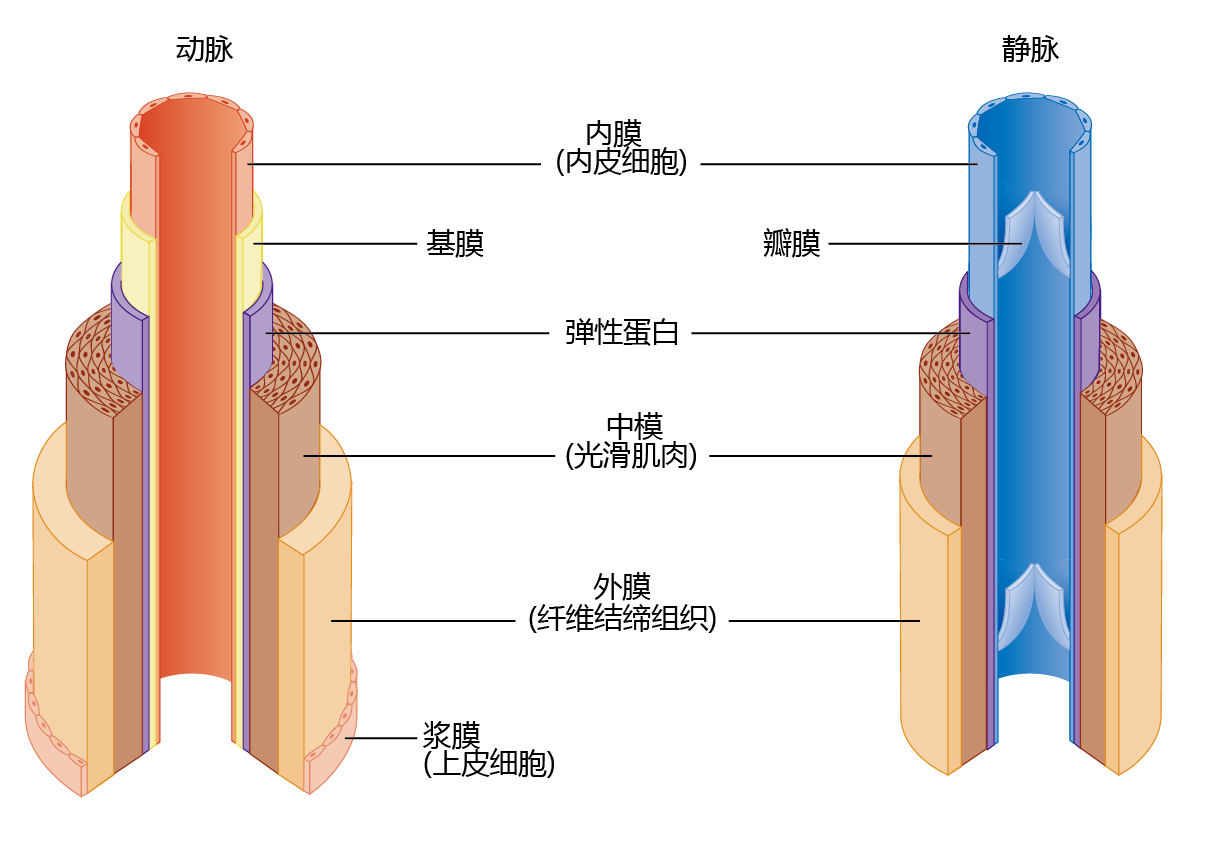
The architecture of arteries is significantly more complex than that of veins, because arteries help maintain blood pressure and flow to the tissue (Figure 9.16). Arteries have a significant amount of circular smooth muscle that, when it contracts, causes a narrowing of the lumen. The smooth muscle responds to neural signals from the brain when systemic blood pressure needs to be increased or blood flow to the extremities needs to be shut down (such as during cold exposure; see Chapter 8). Veins do not use pressure to move the blood back to the heart. Rather, they maintain the flow by one-way valves. When the heart beats and ejects blood, the pressure in the arteries, called systolic pressure, causes the valves to open for blood flow. When the heart is at rest, the drop in pressure in the arteries, called diastolic pressure, causes the valves to close so that the blood in the veins does not flow backward. Skeletal muscle contractions also help veins “pump” blood from areas below the heart. Abnormal age-related alterations in the smooth muscle of the arteries and in the valves of the veins in the extremities result in an age-related disease known as congestive heart failure.
Figure 9.16 Anatomy of arteries and veins. The arteries contain significantly more elastic and connective tissue than the veins. This type of tissue, along with the considerable amount of smooth muscle, has two functions: (1) it allows the arteries to participate with the heart in maintaining blood pressure and flow; and (2) it maintains the structure of the vessels. The connective tissue and smooth muscle of the veins maintain the structure of these vessels.
9.3.2 The heart and arteries are excitable tissues
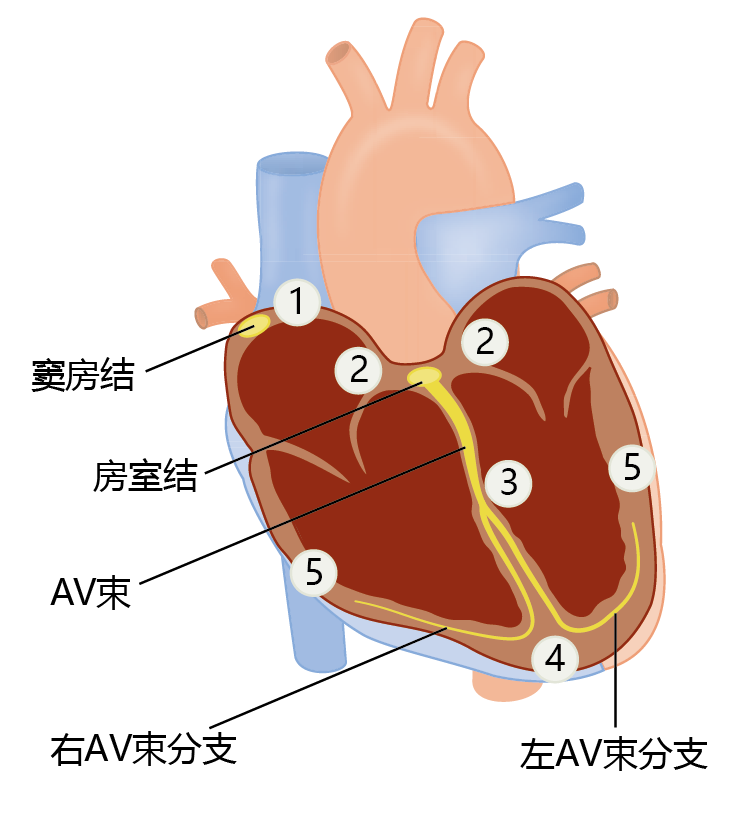
Both the heart and the arteries use the contraction of muscle tissue to generate pressure and flow. Muscle tissue, like nerve tissue, is an excitable tissue that generates action potentials to propagate the contraction from muscle cell to muscle cell. The heart muscle (myocardium) must follow a precise pattern of contraction to operate at peak performance. This pattern begins when the sinus (sinoatrial) node, a small group of specialized cells at the top of the right atrium, generates an action potential (Figure 9.17). The action potential spreads into the adjacent heart muscle cells (myocytes) and causes simultaneous contraction of the right and left atria. The atrioventricular (AV) node picks up the electrical discharge and transmits the signal through the AV bundle and into the AV-bundle branches. Spreading the action potential via the AV node rather than by direct contact between muscle fibers of the atria and ventricles causes a delay of about one-sixth of a second. This delay allows the ventricles to fill with blood prior to contraction. Control of cardiac tissue contraction—the heartbeat—occurs primarily through nerve fibers from the brain stem but can be modulated by hormones such as catecholamines.
Figure 9.17 Electrical conduction in the heart. Electrical conduction follows a pattern that begins with (1) an action potential in the sinus (sinoatrial) node. The action potential spreads simultaneously throughout both atria and into (2) the atrioventricular (AV) node. Propagation of the action potential through the AV node and (3) down the AV bundle branches delays contraction of the ventricles by one-sixth of a second, long enough for the ventricles to fill. As the action potential spreads from (4) the bottom of the ventricles to (5) the top, the high speed of transmission causes all fibers to contract at virtually the same moment.
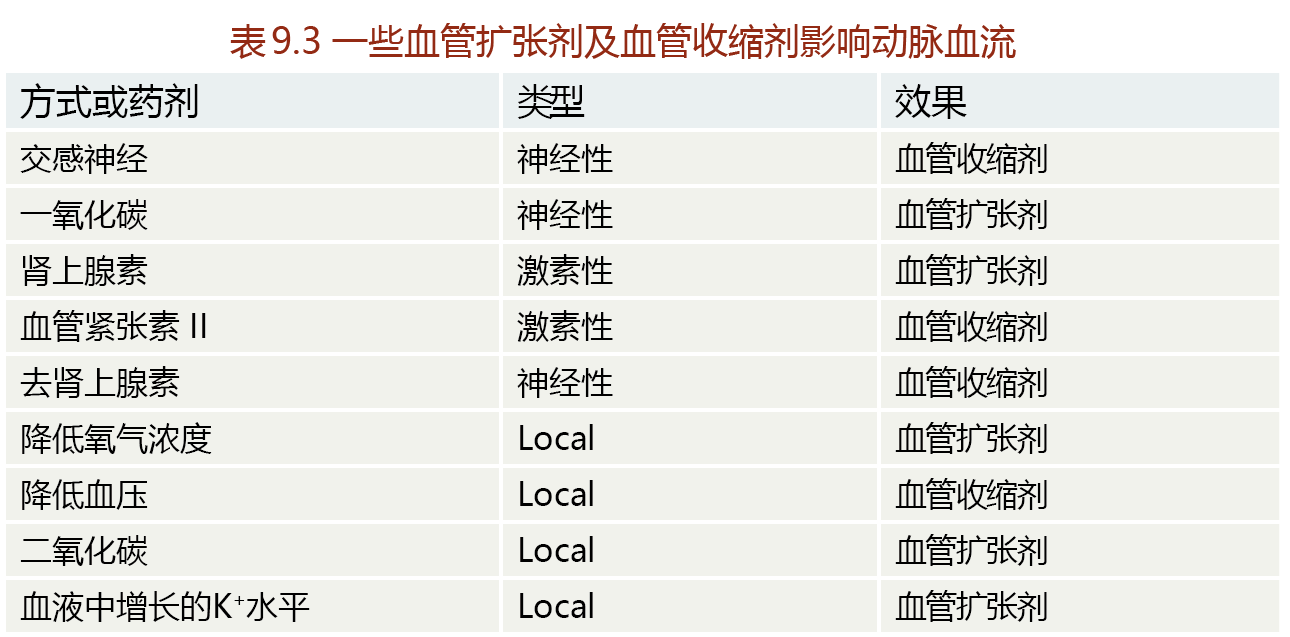
Whereas cardiac muscle contraction is controlled by neural stimulation, contraction of the smooth muscle of the arteries is controlled primarily by hormones and events occurring locally in the arteries (TABLE 9.3). Contraction of the smooth muscle of an artery, called vasoconstriction, results in a decrease in lumen diameter. Relaxation of the smooth muscle, called vasodilation, causes an increase in diameter. We can use the example of an increase in metabolic activity to demonstrate how vasoconstriction and vasodilation work together to supply the necessary blood to an organ. At rest, the arteries of a skeletal muscle are partially vasoconstricted—that is, they have a relatively low need for blood flow. When exercise begins and the muscle begins to contract, catecholamines are released and bind to alpha-adrenergic receptors, causing vasodilation of the arteries (increasing blood flow). This neurohormonal response lasts only for the first few seconds to minutes of the exercise. Vasodilation and increased blood flow during sustained exercise are maintained by local events such as the decreased oxygen content across the arterial-venous bed and the increased blood levels of metabolites of cellular respiration. As exercise comes to a halt, vasoconstricting agents, such as the drop in blood pressure and the binding of catecholamines to beta-adrenergic receptors, reduce blood flow.
9.3.3 The heart controls blood flow and pressure by adjusting cardiac output
The amount of oxygen-rich blood that a heart pumps into the circulation is called cardiac output and can be expressed as the product of the stroke volume, the amount of blood ejected during contraction (systole), and the heart rate, the number of beats per minute (Equation 9.1). Cardiac output corresponds to the sum of all tissue needs. That is, if the tissues need 100 units of blood, then the heart will release 100 units of blood. Tissue needs are determined by the amount of blood returned to the heart from the veins (venous return).
Cardiac output = stroke volume × heart rate
Where
stroke volume = amount of blood (ml) ejected from the left ventricle
during one contraction of the heart (one heartbeat)
heart rate = number of beats per minute
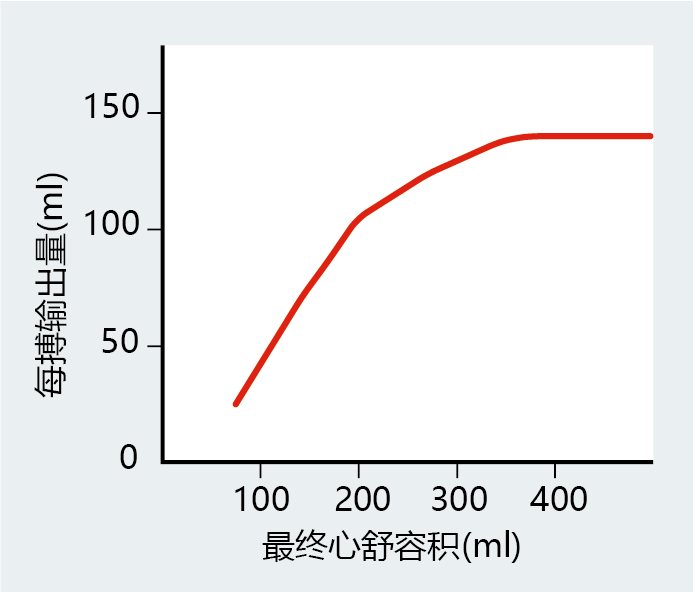
As we begin to move our muscles, such as when we start to climb stairs, neurohormonal factors increase heart rate to supply the body with the extra oxygen needed for muscular work. The increase in cardiac output also causes greater venous return, with more blood filling the ventricles. Stretching of the muscle around the ventricles lengthens the myocytes, a property known as compliance, and causes a greater force of contraction, or greater contractility—that is, more blood can be ejected per beat. Contractility matches compliance over a wide range of end diastolic volumes, the amount of blood in the ventricle before it contracts and a measure of venous return. That is, the amount of blood ejected from the heart matches venous return. However, the myocyte has an optimal length, and any further stretching of the ventricle will decrease its contractility and, in turn, the stroke volume. The lengthtension relationship of compliance and contractility in cardiac muscle, known as the Frank-Starling mechanism, does not operate beyond the optimal myocyte length under normal physiological conditions (Figure 9.18). Later in the chapter, we will see how congestive heart failure can be explained, in part, by the stretching of myocytes beyond their optimal length.
Figure 9.18 Graphical representation of the Frank-Starling mechanism. When venous return increases, so does the end diastolic volume of the ventricles. An increase in end diastolic volume stretches the ventricles, causing the myocytes to lengthen and generate a greater force (increased contractility). In turn, greater contractility generates the necessary force to eject a larger volume of blood. However, the length-tension relationship has its limits; eventually, contractility will begin to fail as myocytes stretch beyond the point at which they can generate the appropriate force of contraction.
9.3.4 Principles of fluid dynamics govern overall blood flow
As we have seen, both the heart and the vascular system have mechanisms that control the volume of blood flow: cardiac output for the heart; vasodilation and vasoconstriction for the arteries. These tissue- and organ-specific mechanisms for blood flow regulation work together to adjust overall blood flow, through some basic principles of fluid dynamics. The most important is the relationship among flow, pressure, and resistance. Blood flow is directly proportional to the pressure difference across the system and is inversely proportional to resistance (Equations 9.2, 9.3, and 9.4). For example, using Equation 9.3, we can see that an increase in either resistance (R) or flow (F) will increase blood pressure (ΔP). Pressure can be returned to its basal level by reducing cardiac output or decreasing vasoconstriction (or increasing vasodilation).
F = ΔP/R (9.2)
ΔP = F × R (9.3)
R = ΔP/F (9.4)
Where
F = blood flow
ΔP = difference in pressure between the two ends of a vessel
R = resistance
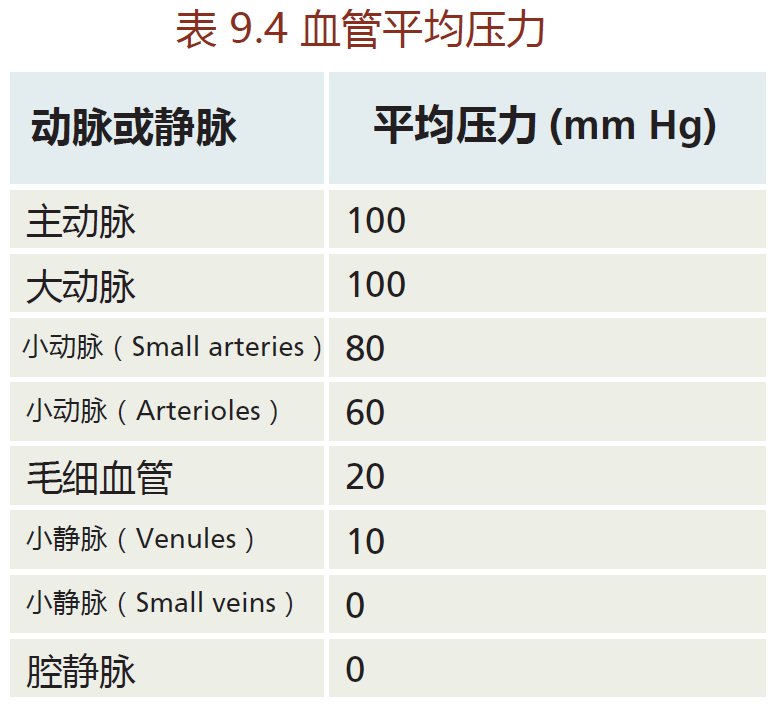
Recall that the arterial vessels get progressively smaller until they reach the capillaries, which are just one red blood cell in diameter. As the vessel gets smaller, pressure decreases (TABLE 9.4) to prevent damage to the vessel. That is, while the large arteries can withstand the 100 mm Hg of pressure that occurs close to the heart, small vessels, especially the arterioles (the smallest arteries) and capillaries, cannot. So how can the cardiovascular system adhere to the laws of fluid dynamics and, at the same time, decrease pressure as the vessel diameter becomes smaller?
The answer lies in the vessel‘s ability to increase its lumen diameter by stretching the wall and taking advantage of another basic physical law of fluid dynamics: the fourth-power law. The fourth-power law states that blood flow in a vessel increases or decreases in proportion to the fourth power of the diameter. This means that small changes in the diameter of a vessel result in large changes in flow. As an example, let's assume that cardiac output has increased, resulting in greater flow to the periphery. The artery wall expands in order to accommodate the increased volume of blood. As the diameter of the lumen increases, resistance decreases, allowing greater flow and the maintenance of blood pressure in the artery. The relevance of the artery‘s ability to increase or decrease its lumen size will become more apparent when we discuss atherosclerosis.