4.6 端粒缩短和复制导致的衰老
In the previous section, we focused our attention on a mechanism in which the random or stochastic phenomenon of oxidative damage results in an accumulation of molecular damage that leads to cellular aging. We now turn our attention to a theory of cell longevity that involves a more programmed approach—the telomere-shortening theory. The telomere-shortening theory predicts that repeated replication of a chromosome shortens the telomeres (the repetitive, noncoding base-pair sequences at the ends of each chromosome) to the point at which no further replication can occur without affecting the coding sequences of the DNA. According to this theory, the DNA repair mechanism “recognizes” the difference between telomeres and coding sequences and halts cell division before the transition from G1 to S phase if genetic information is about to be used to synthesize an RNA primer. That is, the telomere is a biological clock, of sorts, that counts the number of cell replications.
4.6.1 Telomeres prevent the lagging strand from removing vital DNA sequences
Recall that the replication of eukaryotic linear DNA occurs in both directions from the replication origin, with each strand of the original DNA being used as a template for a new copy. Because DNA聚合酶 can synthesize a new strand only in the 5'→3' direction, the lagging strand template must use a backstitching maneuver on the Okazaki fragments to complete the replication of the chromosome. This mechanism requires the synthesis of an RNA primer for each new Okazaki fragment formed. Thus, when DNA聚合酶 reaches the end of the chromosome, there may not be enough DNA available with which to initiate the final RNA primer on the lagging strand template. This is generally referred to as the “end replication problem.”
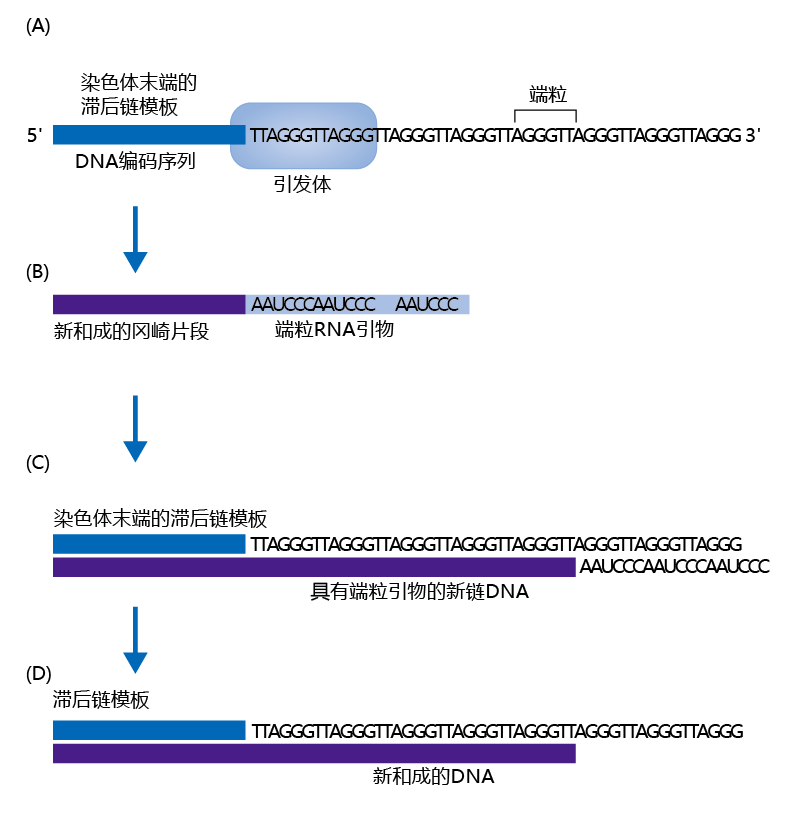
In mitotic eukaryotic cells, telomeres solve the end replication problem. Telomeres are rich in thymine (T) and guanine (G), are highly conserved across the eukaryote kingdoms, and in newly replicated DNA, have a length of 5000–10,000 base pairs. When DNA聚合酶 reaches the end of the coding sequences on the lagging strand template, it uses the telomere as the template for the RNA primer needed to begin the final Okazaki fragment (Figure 4.31) . However, a small number of (10–50) telomere base pairs are lost as a result of the backstitching mechanism. This small loss of telomere sequence during replication prevents the loss of genetic material at the end of the chromosome during replication.
Figure 4.31 How the presence of telomeres solves the end replication problem. (A) The primosome reads the telomere sequence on the lagging strand template at the end of the chromosome, and (B) makes the RNA primer for the next Okazaki fragment. (C) The newly synthesized Okazaki fragment is then joined to the end of the new DNA. (D) Finally, the RNA primer made from the telomere is removed, and the new strand of DNA is complete.
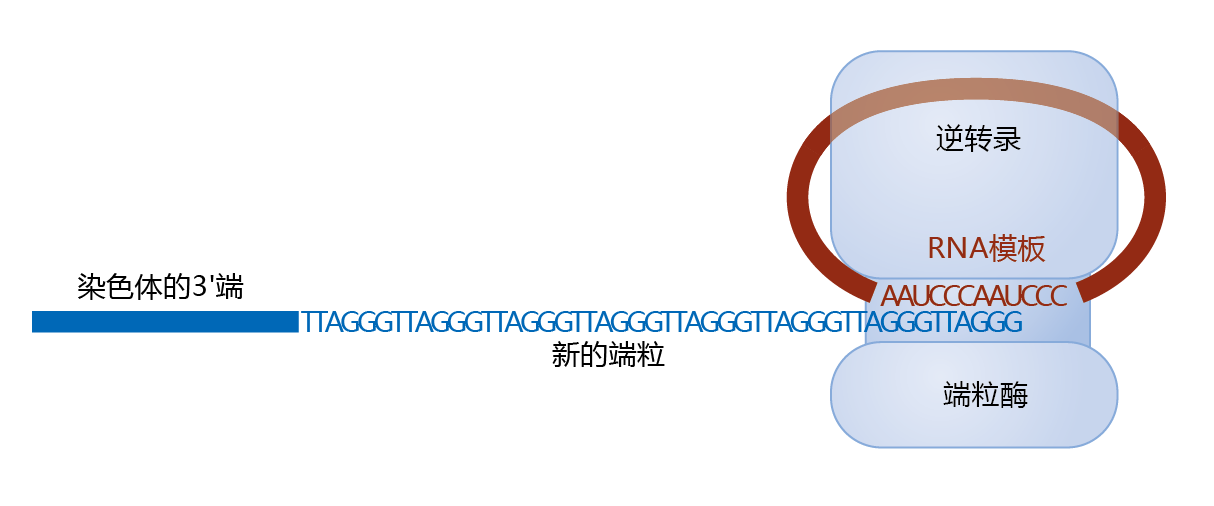
Because replication on the lagging strand template results in the loss of a short segment of the telomeres, repeated cell divisions can result in the complete loss of the telomeres. In actively mitotic cells, however, telomere length is maintained through the action of the enzyme telomerase. 端粒酶has a unique structure that contains both an RNA subunit and a 蛋白质 catalytic subunit (图4.32) . That is, telomerase is an enzyme that carries around its own RNA sequence. The telomerase RNA subunit contains the sequence of the telomeres for that species. In the case of humans, the RNA is approximately 450 nucleotides long, with the repeated sequence AAUCCC (DNA sequence, TTAGGG). The catalytic subunit is a reverse transcriptase (telomerase reverse transcriptase, TERT), an enzyme that reads the RNA sequence, as a template, and synthesizes the corresponding DNA sequence.
图4.32 Telomerase and elongation of the telomere. Prior to the replication of DNA during the S phase, telomerase is attracted to the 3′ end of the lagging strand template. Telomerase contains a reverse transcriptase, a catalytic unit that makes DNA from RNA (designated “reverse” because RNA is usually made from DNA), and the RNA template needed for DNA synthesis.
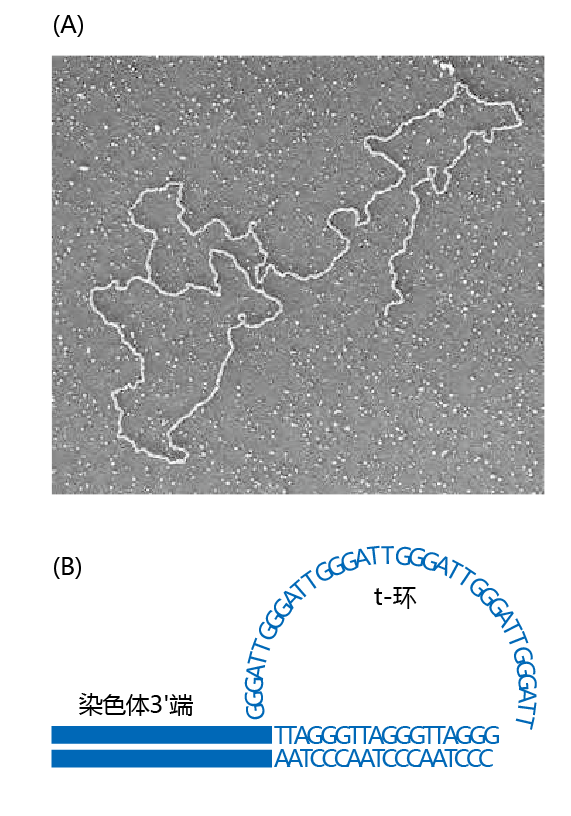
The mechanism of telomere elongation ensures that the 3' end is longer than the 5' end, a condition necessary for appropriate lagging strand replication. Note, however, that if the 3' end were left as is, the DNA repair mechanism could read the extended length at the 3' end as a double-strand break. Telomeres solve this potential problem by forming a loop, called the t-loop, at the 3' end, a procedure known as end-capping, thus “fooling” the DNA repair mechanism into reading it as a double strand(Figure 4.33)
Figure 4.33 Telomere t-loop. (A) Electron micrograph of the t-loop at the end of the chromosome. (B) Illustration of the end-capping of the telomere, a procedure used by the telomere to prevent the possible reading of the 3′ elongation as a DNA double-strand break. (A,from J.D. Griffith et al., Cell 97:503–514, 1999. With permission from Elsevier.)
4.6.2 端粒的缩短可能引起体细胞衰老
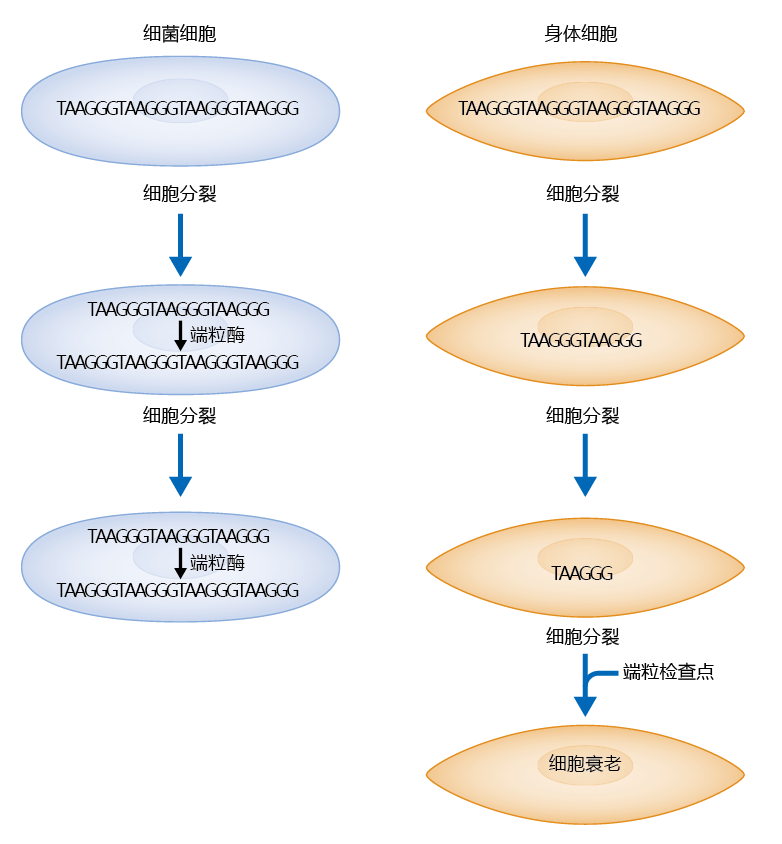
Elongation of the telomeres by telomerase occurs in cells that require high fidelity during replication. That is, telomerase is expressed only in a limited number of cells, which include the germ-line and stem cells. Most somatic cells do not normally express telomerase, so telomere shortening occurs at each replication in these cell types. In vitro experimentation has shown that chromosomes of senescent cells do indeed have shorter telomeres than those observed on the chromosomes of pre-senescent cells. The lack of telomerase in somatic cells and the discovery of short telomeres in senescent cells have led to the widely accepted theory of replicative senescence known as the mitotic clock theory, which predicts that old cells sense short telomeres, which, in turn, causes 细胞周期 arrest (Figure 4.34).
Figure 4.34 The mitotic clock theory of cell senescence. In both germ cells and somatic cells, the telomere shortens after each division. In germ cells, telomerase replaces the telomeres lost during cell replication. Somatic cells do not have telomerase, so the telomere that is lost from the lagging strand is not replaced. In a mechanism that is not well understood, the cells become senescent when the telomere checkpoint identifies critically short telomeres.
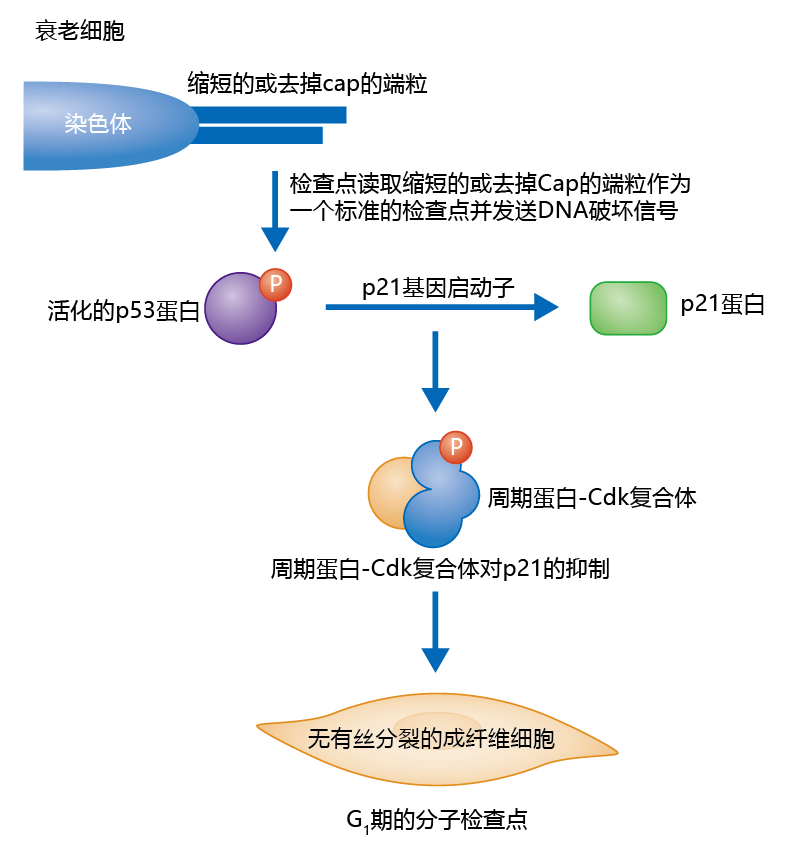
Although the mechanism that underlies the mitotic clock theory has not yet been described, current research suggests that short or uncapped telomeres induce inhibition of the 细胞周期. The inhibition seems to follow the normal cell checkpoint, as the p53 蛋白质 is up-regulated in senescent cells. An example of how senescence may arise as a result of a short or uncapped telomere during G 1 is shown in Figure 4.35. Short or uncapped telomeres arise in somatic cells after several replicative cycles. The DNA damage and repair mechanism reads the telomeres as a strand break and initiates, through various signals, phosphorylation of the p53 蛋白质. Inhibition of the 周期蛋白-Cdk复合体 then halts the 细胞周期 in G 1 . Since there is no repair mechanism that will elongate or cap the telomere—that is, no telomerase—the cell enters G 0 permanently, resulting in replicative senescence.
图4.35 The proposed p53 蛋白质 mechanism of cell senescence. Given enough time and a sufficient number of cell divisions, telomeres in somatic cells (those without telomerase) will shorten or become uncapped. Short or uncapped telomeres are read as strand breaks by the DNA repair mechanism. In turn, signals are relayed to the p53 蛋白质 cascade, the 细胞周期 is halted in G 1 , and the cell enters G 0 permanently.
The mitotic clock theory may also tie predictions about the 进化 bases of aging and longevity to cellular aging. Recall from Chapter 3 that 进化理论 of longevity suggest that the mortality of the somatic cell arose as a result of a trade-off in the use of energy resources between the DNA repair systems of the soma and the germ line. It is easy to see how the shortening of telomeres that leads to cell senescence in somatic cells and the maintenance of telomeres in the germ line fit within the scope of such models. That is, the germ line invests a significant amount of energy in telomerase, to maintain the telomeres and the high fidelity of DNA replication needed for transfer of the genes to the next generation. Somatic cells, on the other hand, invest no resources in telomere lengthening, as they do not express telomerase. Remember that a basic tenet of the disposable soma theory is that the soma need only live long enough to support successful reproduction. It appears, although is not yet proven, that telomeres tell the cells when they have lived long enough.
ESSENTIAL CONCEPTS
- The eukaryotic 细胞周期 consists of four distinct phases: G1, S, G2, and M. An additional phase, G0, indicates that the cell has exited the cycle.
- If a cell does not receive a mitogenic signal at the correct time, it dismantles the 细胞周期 control mechanisms and enters a modified version of the G1 phase known as G0.
- Populations of mitotic cells grown in vitro have a finite life span.
- Senescent cells grown in culture have several common features, including cellular enlargement, increase in the size of the nucleus, multinucleated cells, decreased synthesis of DNA, RNA, and 蛋白质, and increased secretion of inflammatory cytokines.
- Population doubling rates vary with the species and are not strongly correlated with the organism's length of life.
- The mechanism underlying the accumulation of damaged 蛋白质 that causes cellular aging is the disorder of increasing entropy.
- The oxidative stress theory of cellular aging predicts that the intracellular accumulation of damaged biomolecules results from reactions involving oxygen-centered free radicals.
- Oxygen-centered free radicals, or reactive oxygen species (ROS)— •O2− , •OH, and H2O2—arise during normal aerobic metabolism as a result of one-electron reduction of O2 during the synthesis of ATP.
- The double-bond structure of the polyunsaturated fats found in the lipid component of cell membranes is particularly susceptible to “attack” by the hydroxyl radical. This “attack” causes a chain reaction that propagates additional free radicals and alters the polyunsaturated fat structure to form a new molecule, a lipid peroxide.
- ROS can also have beneficial effects for the aerobic organism. Macrophages of the immune system use ROS to break down bacterial membranes. ROS generated during oxidative metabolism may also induce the expression of cellular antioxidants such as SOD and catalase.
- The telomere-shortening theory predicts that repetitive replication of the chromosome shortens the telomere to the point at which no further replication can occur without affecting the coding sequences of the DNA.
- The repeating sequences of telomeres at the ends of chromosomes solve the end replication problem.
- In highly mitotic cells, telomere length is maintained by the enzyme telomerase.
- The mitotic clock theory predicts that cell senescence occurs when old mitotic cells sense short telomeres, which, in turn, causes 细胞周期 arrest at the G1 -S phase transition.
本章结束