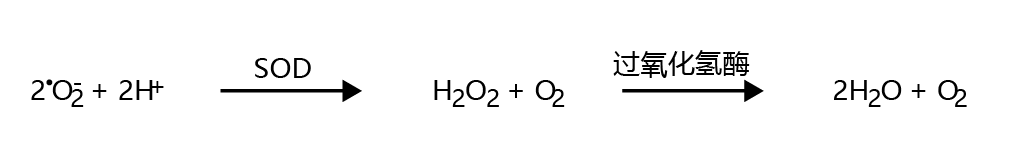4.5 氧化压力和细胞衰老
Somewhere between 2.5 and 3 billion years ago, cells of blue-green algae (cyanobacteria) began to convert atmospheric carbon dioxide (CO2) into glucose, using the energy of sunlight. During this process, known as photosynthesis, oxygen (O2) was released into the atmosphere. As the O2 began to accumulate, two critical events occurred that led to a greater diversity of life. First, the radiant energy of the sun converted O2 into ozone (O3), which accumulated in the upper atmosphere and trapped harmful ultraviolet rays. Life was now able to leave the protection of the oceans and take up residence on land. Second, because oxygen-based metabolism, known as aerobic metabolism, is much more efficient than metabolism without oxygen (anaerobic metabolism), single-cell organisms that used aerobic metabolism grew bigger and competed more successfully for the available resources. The success of these 单细胞 aerobic organisms over anaerobic cells led to the 进化 of 多细胞生物。
Unfortunately for many organisms, the accumulation of oxygen in the atmosphere was deadly. The molecular structure of O2 makes it extremely toxic to organisms that cannot safely reduce the by-products of aerobic metabolism to water. Organisms without protection from oxidation would soon die off, leaving only those that could defend themselves from the harmful effects of O2. In other words, organisms with the highest fitness were those that could use O2 for their benefit.
In 1956, Denham Harman theorized that as cells age, some by-products of aerobic metabolism known as oxygen-centered free radicals, products of oxygen metabolism having one or more unpaired electrons, escape the normal degradation pathways and cause damage to biomolecules. In turn, these damaged molecules accumulate in the cell and lead to cellular aging. His theory, the oxidative stress theory, was once thought to be the primary mechanism of aging. We now know that oxidative stress is but one of many mechanisms that cause damage to a cell that leads to age-related dysfunction, but damage caused by oxidative stress remains one of the most heavily researched areas in biogerontology. Because of the vast amounts of information on damage caused by oxygen stress, we are using this process as one example of a random event that leads to the accumulation of cell damage. This process should not be seen as the primary or the only cause of cellular damage leading to aging.
4.5.1 Oxidative metabolism creates reactive oxygen species
Before explaining the nature of oxygen-centered radicals, it might be helpful to briefly review oxidation-reduction reactions that convert organic fuels into cellular energy. The flow of electrons from one compound to the next forms the basis of how we create usable cellular energy from the nutrients we ingest—fats, carbohydrates, and 蛋白质—and determines the reactivity of a compound. A substance is oxidized when it loses one or more electrons; it is reduced when it gains one or more electrons. In any reaction involving electron flow between two substances, oxidation and reduction have to work together. When one substance loses an electron (oxidation), the other substance gains an electron (reduction). Oxidized compounds tend to be highly reactive, because they are seeking electrons from other compounds to stabilize their electron configuration. Reduced compounds tend to be more stable than oxidized compounds, because their electron configuration is closer to the ground state.
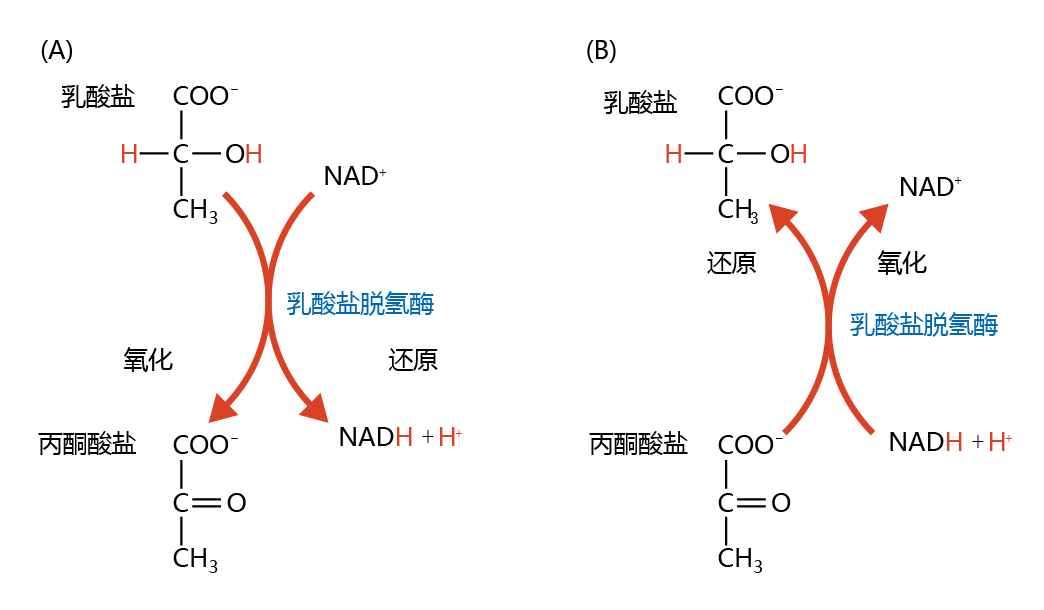
Oxidation-reduction in biological processes can be characterized by the loss or gain of oxygen and hydrogen. If a compound gains oxygen or loses hydrogen, it has been oxidized. If it loses oxygen or gains hydrogen, it has been reduced. In biochemistry, oxidation-reduction of compounds is usually associated with a third molecule in processes known as coupled oxidation-reduction reactions. Figure 4.14 illustrates the coupled oxidation-reduction involving pyruvate and lactate. Lactate is oxidized (loss of hydrogen) to form pyruvate through the reduction (gain of hydrogen) of nicotinamide adenine dinucleotide (NAD+) to NADH+ H+. Conversely, pyruvate is reduced to lactate when NADH+ H+ is oxidized to NAD+.
Figure 4.14 Coupled oxidationreduction reactions involving pyruvate and lactate. (A) In the oxidation of lactate to pyruvate, two hydrogens (red) of lactate are stripped by the oxidized form of nicotinamide adenine dinucleotide (NAD+), creating the reduced form, NADH+ H+. (B) Conversely, pyruvate is reduced to lactate with the addition of two hydrogens from the reaction involving the oxidation of NADH+ H+ to NAD+.
This brief description of oxidation-reduction reactions might also help you to understand how antioxidants work, an important concept in radical chemistry. Recall that oxidized compounds have fewer electrons than reduced compounds, making them more reactive. An antioxidant is a compound that donates electrons to an oxidized molecule, making it more reduced and less reactive. You will learn in this section how antioxidants such as catalase, vitamin E, and vitamin C protect the cell from oxidized compounds and radicals.
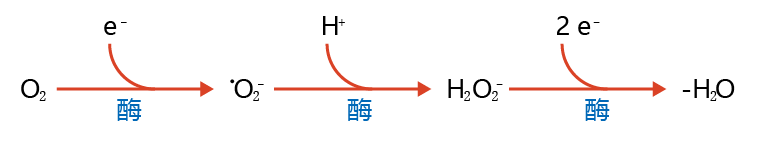
Oxygen-centered free radicals, also commonly known as reactive oxygen species (ROS), include the superoxide radical (•O2−), hydrogen peroxide (H2O2), and the hydroxyl radical (•OH). Although the ground state of diatomic oxygen (O2) is classified as a radical species, it is only mildly reactive. Oxygen has two unpaired electrons in different orbits that have matching spins. Therefore, O2 can react only with other atomic species that have two unpaired electrons with spin antiparallel to that of the unpaired oxygen electrons, a rare occurrence in nature. As a result, the reduction of O2, a vital process in aerobic metabolism, must occur one electron at a time. One-electron reduction of oxygen results in a highly reactive species with a single unpaired electron (remember what you read above about the loss of electrons and reactivity), the superoxide radical (•O2−); an additional one-electron reduction of •O2− results in the equally destructive hydrogen peroxide (H2O2) molecule. Aerobic organisms have developed enzymatic mechanisms that allow for the full reduction of oxygen to harmless compounds such as H2O (Figure 4.15). Reactive oxygen species and their reduction can occur in different parts of the cell, including the mitochondria, nucleus, and cytosol.
Figure 4.15 General scheme of the enzymatic reduction of oxygen (O2) to water (H2O). During normal aerobic metabolism, oxygen is reduced by one electron to form the superoxide radical (•O2−). Almost instantaneously, enzymes catalyze the reduction of •O2− to hydrogen peroxide (H2O2 ). In turn, other enzymes catalyze a two-electron reduction of H2O2 to form H2O.
4.5.2 线粒体的ATP合成产生了主要的过氧化物离子
The mitochondria of aerobic organisms (Figure 4.16) use 95% of all oxygen consumed by the body for the process of synthesizing adenosine triphosphate (ATP). This molecule, shown in Figure 4.17, is the primary molecule that supplies chemical energy for cellular reactions. In its simplest form, oxidative metabolism converts the energy stored in the carbon-carbon bonds of energy nutrients—carbohydrates and fats (蛋白质 are rarely used for energy in aerobic organisms)—into potential usable energy in the form of ATP. The conversion of ATP to adenosine diphosphate (ADP) releases the energy that drives many biochemical reactions.
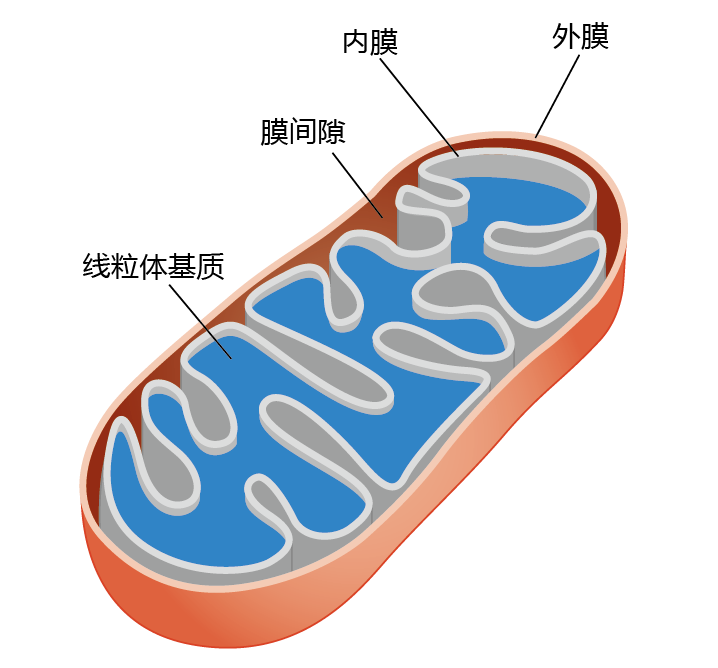
Figure 4.16 The mitochondrion. The outer membrane separates the mitochondrion from the cytosol and contains enzymes associated with fat metabolism. The inner membrane is folded in order to increase the surface area for the reactions associated with electron transport and ATP synthesis. The matrix contains enzymes for oxidative phosphorylation, as well as the mitochondrial DNA. The intermembrane space contains the enzymes necessary for the transport of ATP out of the mitochondrion.
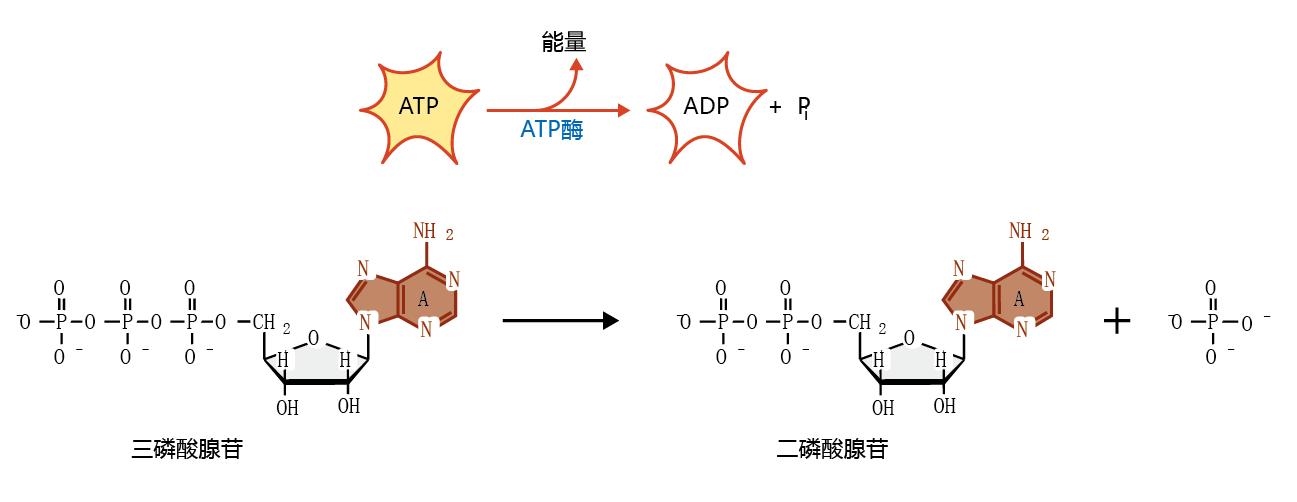
Figure 4.17 Structure of adenosine triphosphate (ATP) and adenosine diphosphate (ADP). The release of energy from the breaking of one phosphate bond in the conversion of ATP to ADP supplies the majority of chemical energy for cellular metabolism.
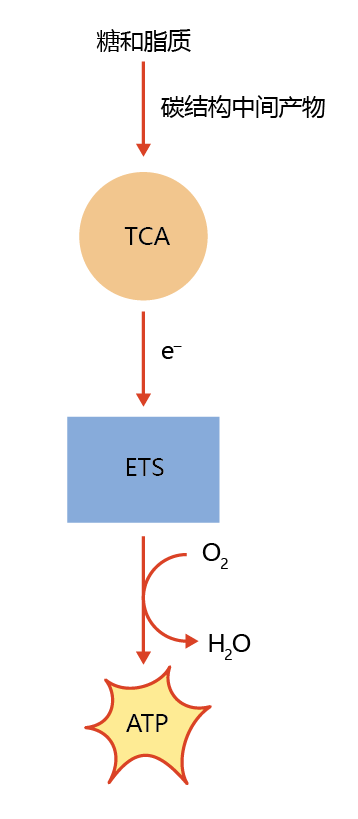
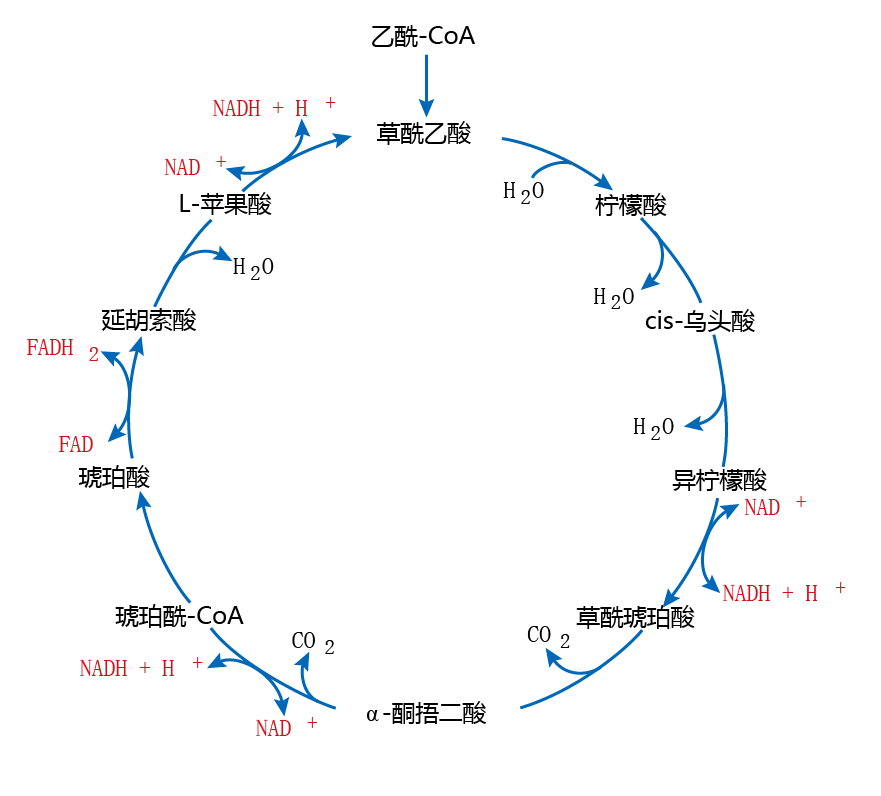
The transfer of energy from nutrient substrates to ATP occurs in two distinct but coupled systems within the mitochondria. The first system generates electrons through a series of oxidation-reduction reactions as part of the tricarboxylic acid cycle (TCA cycle), also known as the Krebs cycle and the citric acid cycle. The TCA cycle is located in the matrix of the mitochondria. The TCA cycle releases electrons from the carbon-carbon bonds through a cascade of enzyme-catalyzed oxidations. These electrons are then “shuttled” to the second system, the electron transfer system (ETS), located in the 线粒体内膜。The ETS uses these electrons, through a series of enzymecatalyzed reductions, to provide the necessary energy for ATP 合成(Figure 4.18). The overall process is called oxidative phosphorylation. As shown in Figure 4.19, oxidative phosphorylation begins when carbons from nutrient substrates—carbohydrates and fats—are used to generate the molecule acetyl-CoA. At specific points in the TCA cycle, compounds called electron (energy) carriers or reducing equivalents, such as nicotinamide adenine dinucleotide (NAD+) and flavin adenine dinucleotide (FAD), “pick up”the electrons released in the oxidation reactions. These electron carriers deliver the electrons to the ETS.
Figure 4.18 Production of cellular energy (ATP) from food energy (carbohydrates and lipids). The potential energy in the carbon-carbon bonds of carbohydrates and lipids cannot be used directly by the cell. Rather, through the reduction of carbon-containing intermediates in the tricarboxylic acid (TCA) cycle, electrons are released and react with oxygen in the electron transfer system (ETS), with the synthesis of adenosine triphosphate (ATP).
Figure 4.19 The tricarboxylic acid (TCA) cycle. Food substrates (carbohydrates and fats) are oxidized to acetyl-CoA, which can enter the TCA cycle. In a series of oxidation-reduction (redox) reactions, electrons are generated and shuttled to the electron transfer system (see Figure 4.20) by the electron carriers NADH+ H+ and FADH 2 (shown here in red).
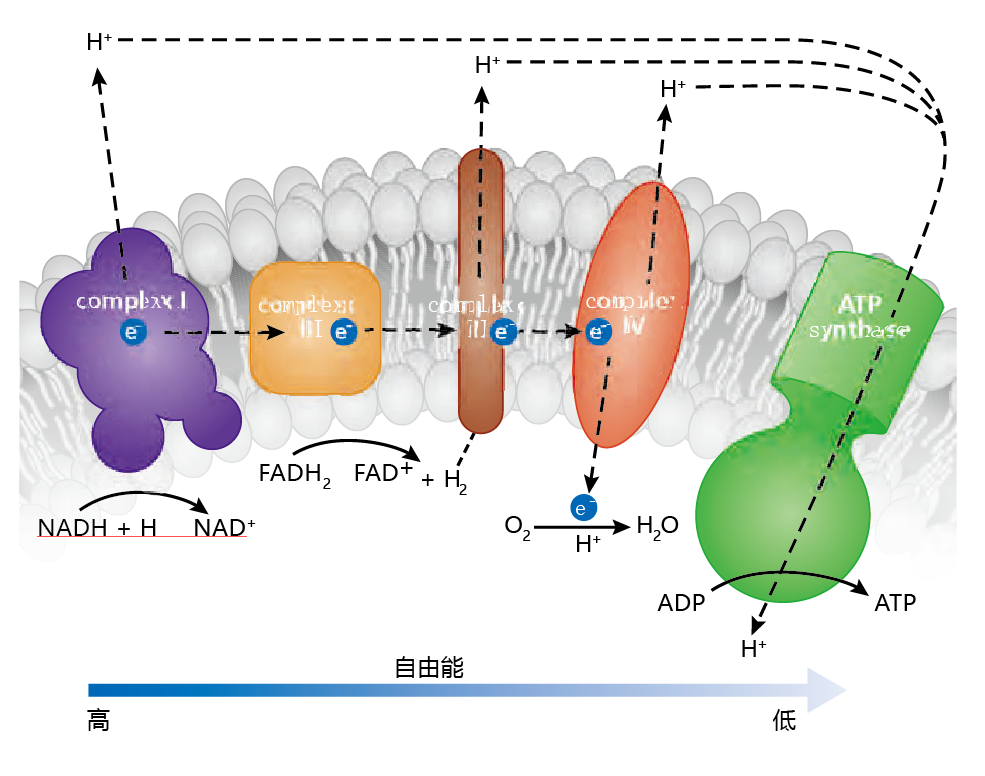
In understanding how the ETS aids in the synthesis of ATP, it can be helpful to think in terms of physics and to remember three important points: (1) the free energy associated with electrons drives the system; (2) a proton (H+) gradient must be established between the mitochondrial matrix and the intermembrane space; (3) the concentration of protons in the intermembrane space must be greater than the concentration of protons in the matrix. The electrons generated in the TCA cycle are shuttled to the ETS by the reducing equivalents, NADH+ H+ and FADH2, and released through oxidation reactions(Figure 4.20) . The protons from these reactions are “pumped” through the inner membrane into the intermembrane space by specialized 蛋白质. The use of these specialized 蛋白质 is necessary because the inner membrane is impermeable to protons. The impermeability of the inner membrane is required to establish the proton gradient. Electrons provide the free energy that drives the pumps. As the electrons give up their free energy, the proton gradient becomes greater. As the electrons are transferred within the ETS, the free energy maintaining the gradient decreases and protons flow back into the matrix. But this flow can happen at only one site on the membrane, the enzyme ATP synthase, which catalyzes the reaction that makes ATP.
Figure 4.20 A simplified version of the electron transfer system (ETS) and the synthesis of ATP. The high energy of electrons drives the pumping of protons by the three respiratory complexes into the intermembrane space and establishes the proton gradient. In the process, the free energy decreases and protons (H+) flow back through ATP synthase, providing the energy needed to synthesize ATP. The free energy left after ATP synthesis is dissipated when oxygen accepts an electron and proton in a reaction that produces water.
The energy state of any system will strive to reach equilibrium. Therefore, even the low free-energy state of the mitochondrial matrix must be dissipated. It is here that oxygen has its role. Oxygen acts as the final acceptor of free energy in the form of electrons and protons, producing water. While this system is very efficient in terms of the complete reduction of oxygen to water, some oxygen will be reduced by only one electron, and a superoxide radical will be generated.
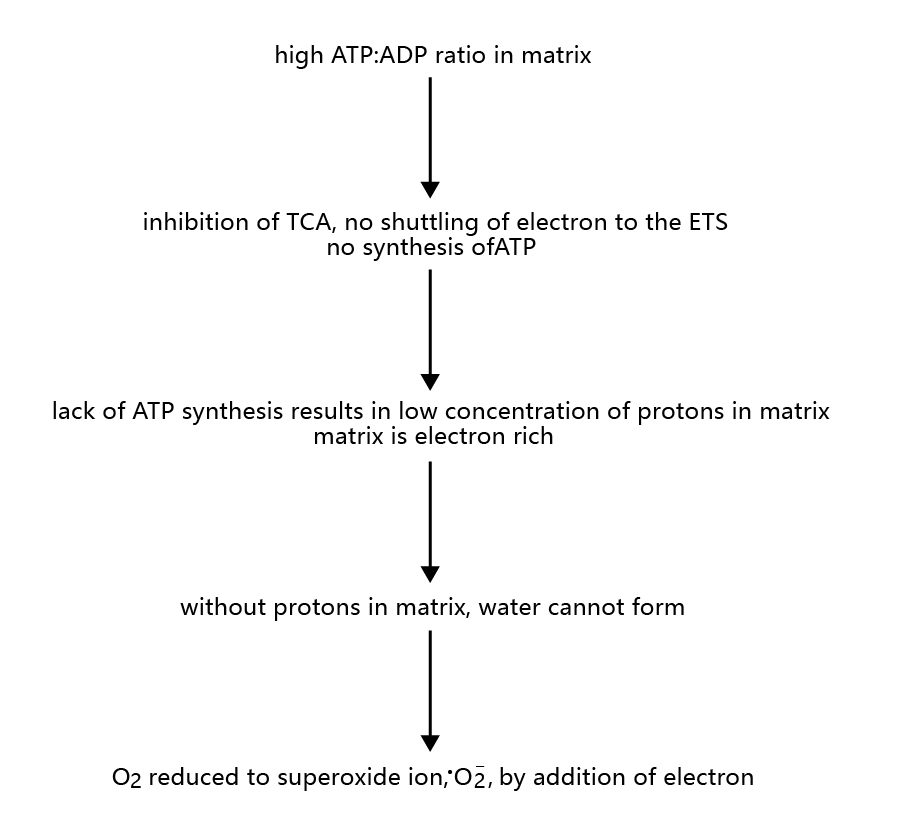
The coupling of the TCA cycle to the ETS is extremely efficient with respect to the use of oxygen as the final electron acceptor during ATP synthesis. That is, very few, if any, superoxide radicals are generated when the TCA cycle is active. However, when the rate of ATP synthesis is low, during periods when the ATP-ADP ratio is high in the mitochondrial matrix, the conditions in the ETS are favorable for the generation of the superoxide radical, • O2−(Figure 4.21) . High ATP-ADP ratios in the mitochondrial matrix inhibit the TCA cycle, and electrons are not shuttled to the ETS. Protons are not flowing through ATP synthase, and thus O2 is not being reduced to water. The low concentration of protons in the matrix inhibits the formation of water from reduced oxygen. Therefore, oxygen can become reduced by one electron, donated from the electron-rich (reduced) ETS complexes, forming •O2−. Estimates suggest that 1–2% of all cellular oxygen consumption results in the superoxide radical.
Figure 4.21 Steps in the generation of the superoxide radical, •O2–, in mitochondria.
4.5.3 Enzymes catalyze reduction of the superoxide radical to water
As described above, • O 2 − can be generated during normal aerobic metabolism. Left unattended, these radicals react quickly with other atoms, leading to the possibility of cellular damage. Fortunately for aerobic organisms, the mitochondria contain two enzymes that catalyze the reduction of •O2− to water: superoxide dismutase (SOD) and catalase. Superoxide dismutase has a very high affinity for •O2−, resulting in immediate reduction of this radical to H2O2 (Figure 4.22). However, H2O2 can also cause significant harm to the cell. The second enzyme in the reduction cascade, catalase, performs a two-electron reduction of H2O2 to form H2O.
Figure 4.22 Reduction of the superoxide radical to water in mitochondria. Superoxide dismutase (SOD) reduces two superoxide radicals to hydrogen peroxide (H 2 O 2 ). Catalase then reduces hydrogen peroxide to water.
4.5.4Cytosolic reduction also generates free radicals
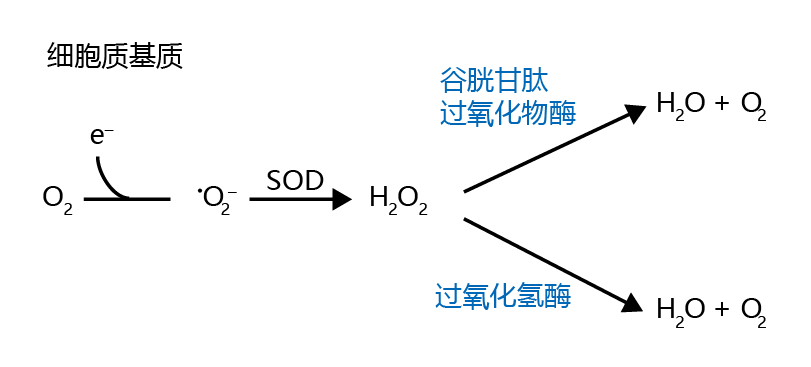
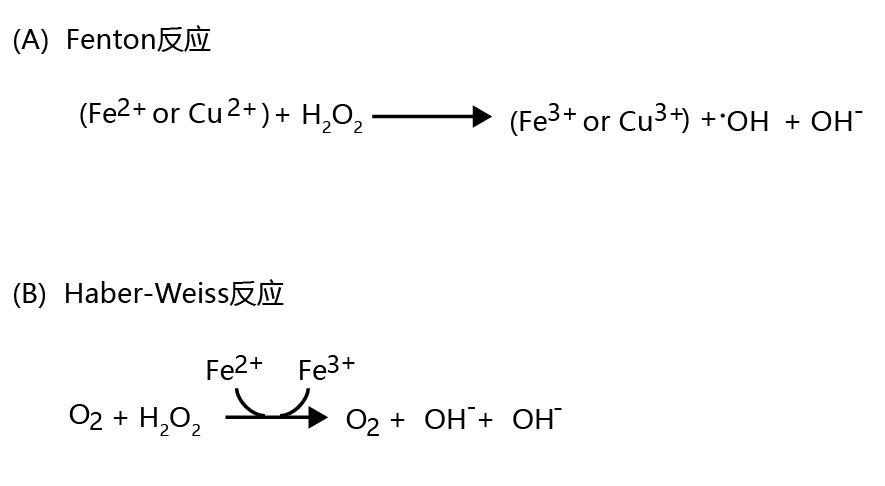
Although the oxygen concentration in the cytosol is considerably lower than that in the mitochondria, superoxide ions can still be generated from a single-electron reduction. When this occurs, cytosolic •O2− is reduced to water by superoxide dismutase and catalase, a reaction similar to that occurring in the mitochondria (shown in Figure 4.22). Cytosolic H2O2 can also be reduced to water with the aid of the enzyme glutathione peroxidase(Figure 4.23). However, under certain cytosolic conditions, hydrogen peroxide is converted to the highly reactive hydroxyl radical, •OH. This conversion occurs nonenzymatically when (1) ferric iron or cupric copper (Fe2+ or Cu2+ ) and H2O2 are present together in the cytosol, a reaction known as the Fenton reaction (Figure 4.24A) ; and when (2) Fe 2+ , H2O2, and •O2− are present together in the cytosol, a process known as the Haber-Weiss reaction (Figure 4.24B).
Figure 4.23 Cytosolic reduction of the superoxide ion to water. The reduction of O2 to •O2− is followed by reduction of the superoxide to H2O2 by cytosolic SOD. The hydrogen peroxide is then reduced to H2O and O2 by catalase or glutathione peroxidase.
Figure 4.24 Generation of the hydroxyl radical (•OH) in the cytosol. Hydrogen peroxide can be converted to the highly reactive hydroxyl radical, •OH, in (A) the Fenton reaction and (B) the Haber-Weiss reaction. Both reactions proceed nonenzymatically.
4.5.5 Oxygen-centered free radicals lead to the accumulation of damaged biomolecules
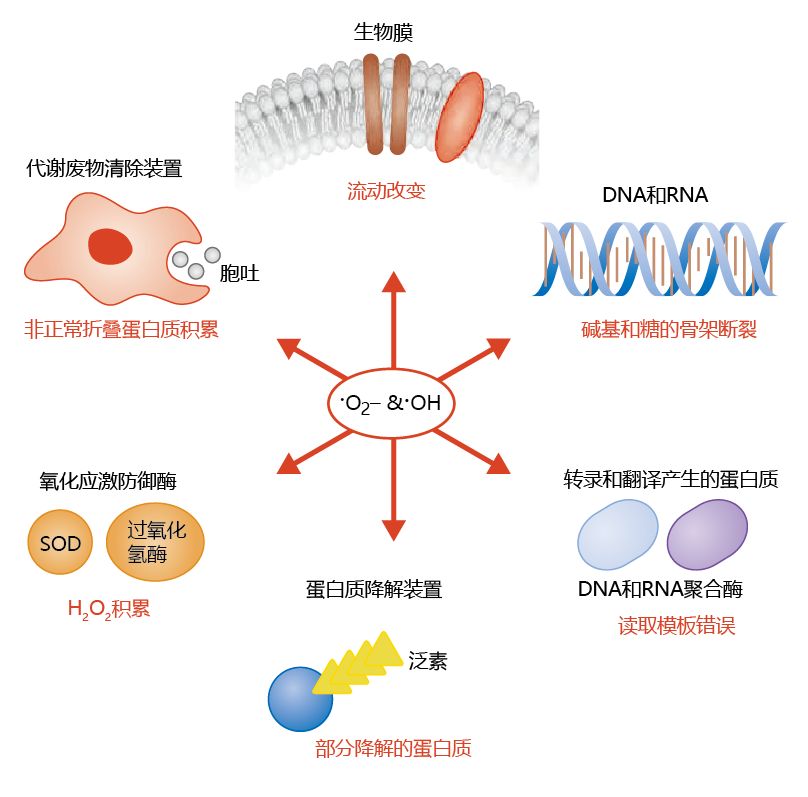
Radicals are highly reactive and cause significant alterations in the structure of many biological molecules, such as nucleic acids, lipids, and 蛋白质. In turn, the alteration in structure leads to a reduction in the molecule's biological activity. This damage can be observed at all levels of cellular organization(Figure 4.25) . As you will see, the accumulation of lipid peroxides in membrane phospholipids renders the cellular and mitochondrial membranes less effective at maintaining the barrier between the extracellular and intracellular compartments. In turn, chemical reactions that are sensitive to the concentration of solutes and water are affected. Oxidative damage to components of the DNA transcription and translation process results in 蛋白质 with altered amino acid sequences. Moreover, because ROS can also affect the 蛋白质 of the DNA repair mechanisms, inappropriate base-pair substitutions may not be removed and replaced with the proper base. It seems that 蛋白质 involved in the removal of damaged 蛋白质 from cell are also affected by ROS. That is, accumulation of damaged 蛋白质 is exacerbated by a decline in the function of waste removal by the cell.
Figure 4.25 Reactive oxygen species can lead to the cellular accumulation of damaged biomolecules at all levels of cellular organization.
Although 线粒体generate the majority of cellular ROS as •O2− ,the efficiency of the mitochondrial antioxidant system (SOD and catalase) results in very few superoxide ions to cause damage. Rather, the ROS of the cytosol (H2O2 and •OH) seem to be responsible for most of the cellular damage caused by free radicals, although they account for only 15% of the total cellular ROS. This reflects the fact that •OH reacts very quickly—in 1 × 10−12 seconds—with polyunsaturated fats, compounds found throughout the cell but primarily in 生物膜(see the discussion below).
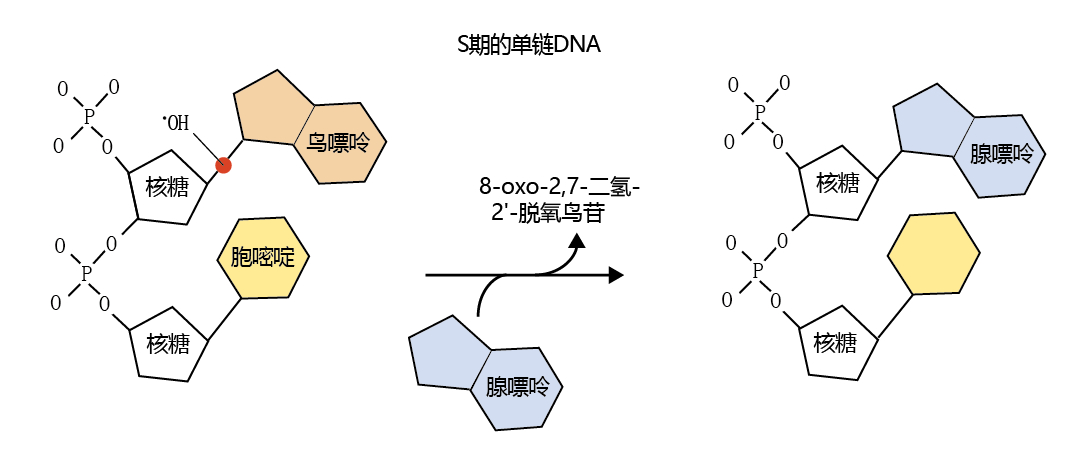
Oxygen-centered radicals can also result in an alteration DNA的碱基对序列. While most research has found that in senescent cells, DNA errors due to damage from radicals are rare, the hydroxyl radical (•OH) does have a high affinity for the bond between the sugar backbone of DNA and guanine (Figure 4.26) . If, for example, the error occurs during the replication process, adenine may be substituted for guanine. The substitution of adenine for guanine would result in an A-T pairing rather than a G-C pairing during DNA replication. In turn, the resulting 蛋白质 would be assembled with an incorrect amino acid sequence, which could lead to decreased biological activity or accumulation of damaged 蛋白质.
Figure 4.26 Effect of the hydroxyl radical (•OH) on DNA. The hydroxyl radical, shown here as a red dot, can break the bond between guanine and the sugar backbone of DNA during replication. If the repair mechanism of DNA does not recognize the substitution, the replicated DNA will be out of sequence. The by-product of the base-pair break, 8-oxo-2,7-dihydro-2 ′ -deoxyguanosine, can be used as a measure of DNA damage.
4.5.6 Cell membranes are susceptible to damage by reactive oxygen species
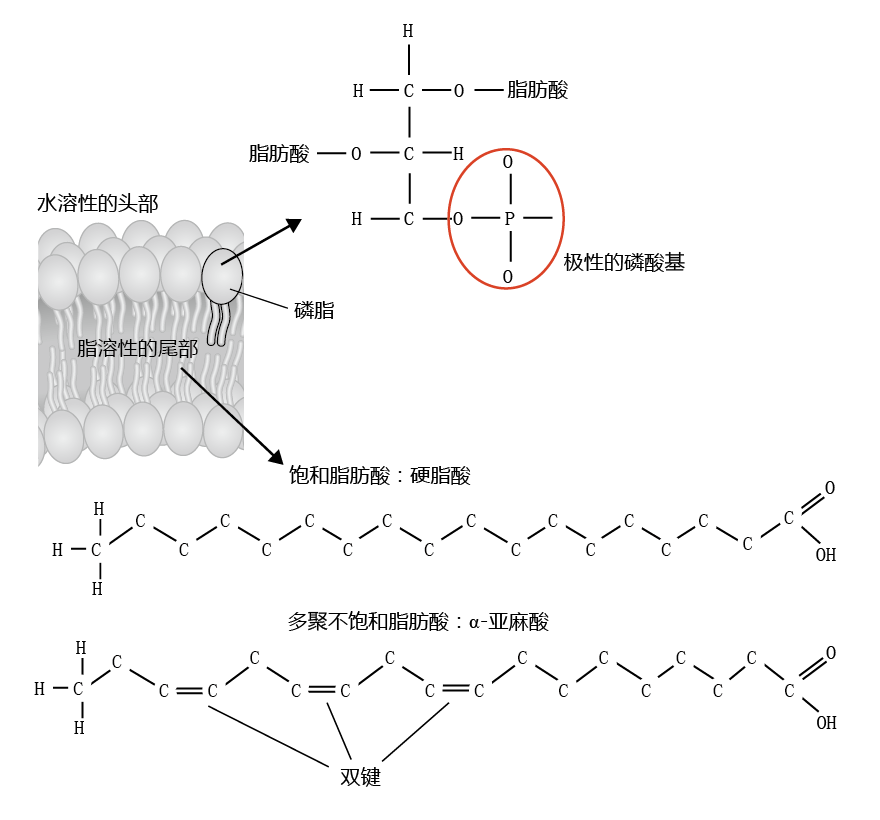
Cell membranes maintain a highly efficient barrier between the intracellular and extracellular compartments, while at the same time allowing an exchange of molecules essential for cellular functions. The unique properties of cell membranes are due to the chemical structure and physical arrangement of fatty acids (lipids) and the various molecules embedded in the membranes. The membrane lipids are known as phospholipids, because they contain a water-soluble phosphate head and two water-insoluble fatty acid tails (Figure 4.27). Cell membranes are also known as lipid bilayers, because the phospholipids are arranged in a double layer, such that the phosphate ends of one layer of phospholipids point toward the extracellular space, while the phosphate ends of the other layer point toward the intracellular space. The lipid components of the phospholipids point toward each other within the interior of the membrane. This arrangement ensures that water and water-soluble molecules do not pass freely between the extracellular and intracellular spaces, an important property for maintaining proper chemical balance of the cell. Water-soluble molecules are transported into or out of the cell by specialized structures embedded in the membrane.
Figure 4.27 Structure of the phospholipid found in cell membranes. The polar phosphate group of the phospholipid head is water-soluble, or hydrophilic. The fatty acid tails are water-insoluble, or hydrophobic. The phospholipid can contain two saturated fatty acids, two unsaturated fatty acids, or one of each. Most phospholipids found in biological membrane have one of each: a saturated and an unsaturated fatty acid.
A highly functioning membrane is dependent, in large part, on the physical arrangement of the phospholipids and the structures embedded in the membrane. If adhesion between the structures in the membrane and the phospholipids is too tight, water-insoluble molecules cannot pass freely through the membrane. If adhesion is too loose, an inappropriate amount of water and water-soluble molecules can enter or escape from the cell. Thus, the bilayer membrane has evolved so that the lipids and 蛋白质 are in perfect balance to achieve the proper electrochemical composition for just the right membrane structure. Any change in that balance will alter membrane function and thus cellular function. Alterations in biomembranes caused by ROS can lead to disruption of membrane permeability, imbalance of the electrolyte gradient, inhibition of normal membrane 蛋白质 mobility, and disruption in a variety of other functions critical to normal cellular activity.
The fluid nature of the cell membrane reflects, in large part, the bonding characteristics of the fatty acids contained in the phospholipids. Phospholipids are a mixture of saturated and unsaturated fatty acids that combine to give the membrane a particular viscosity. Saturated fatty acids have greater viscosity at body temperature than do unsaturated fatty acids. Thus, combining the two into phospholipid imparts a fluidity to the membrane that results in optimal function.
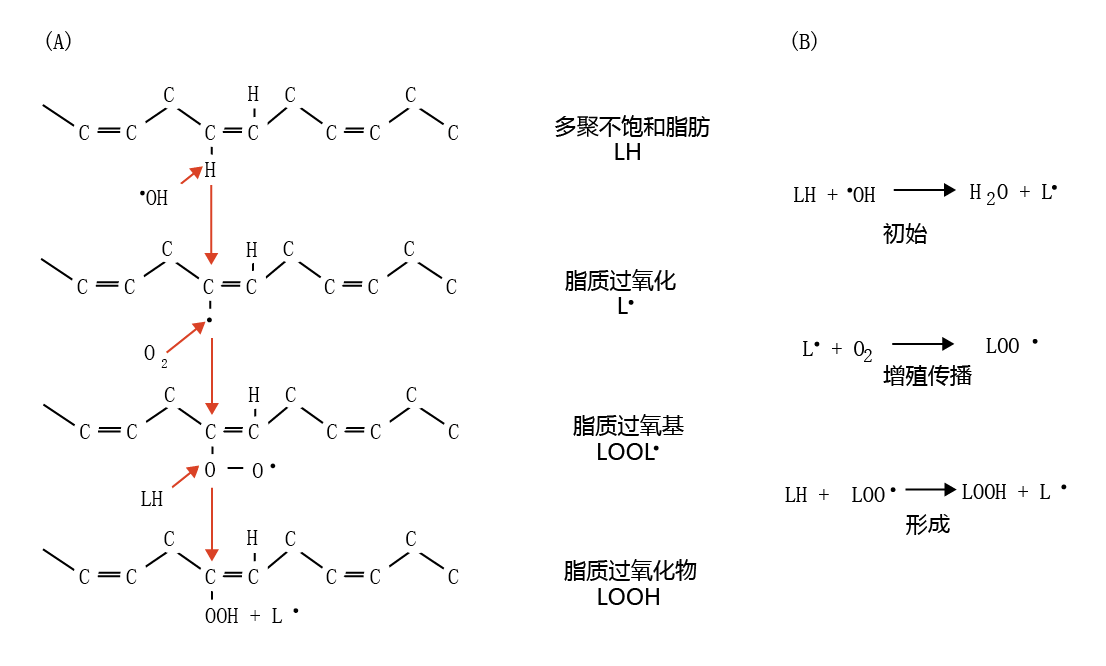
The double-bond structure of the polyunsaturated fats found in the lipid component of cell membranes is particularly susceptible to “attack” by •OH. The •OH “attack” on polyunsaturated fats causes a chain reaction that propagates additional free radicals and alters the polyunsaturated fat to form a new molecule, a lipid peroxide. As shown in Figure 4.28, the •OH donates its unpaired electron to a double bond of a polyunsaturated fat (LH), forming a lipid radical (L•) and water. This reaction marks the initiation phase of the free-radical chain reaction. Cytosolic O 2 is reduced by the L•, generating the lipid peroxyl radical (LOO•), which, in turn, attacks another LH, resulting in L • and lipid peroxide (LOOH) and the further generation of LOO•, LOOH, and L•.
Figure 4.28 Formation of lipid peroxide. (A) The hydroxyl radical “attacks” the polyunsaturated fat (LH) at a double bond, resulting in formation of the lipid radical (L•). In turn, cytosolic O2 reacts with the unpaired electron of the lipid radical to form the lipid peroxyl radical (LOO•). The lipid peroxyl radical attacks another polyunsaturated fat, forming the lipid peroxide molecule (LOOH) and a new lipid radical, and so on. (B) Initiation and propagation of the free-radical chain reaction leading to the formation of lipid peroxide.
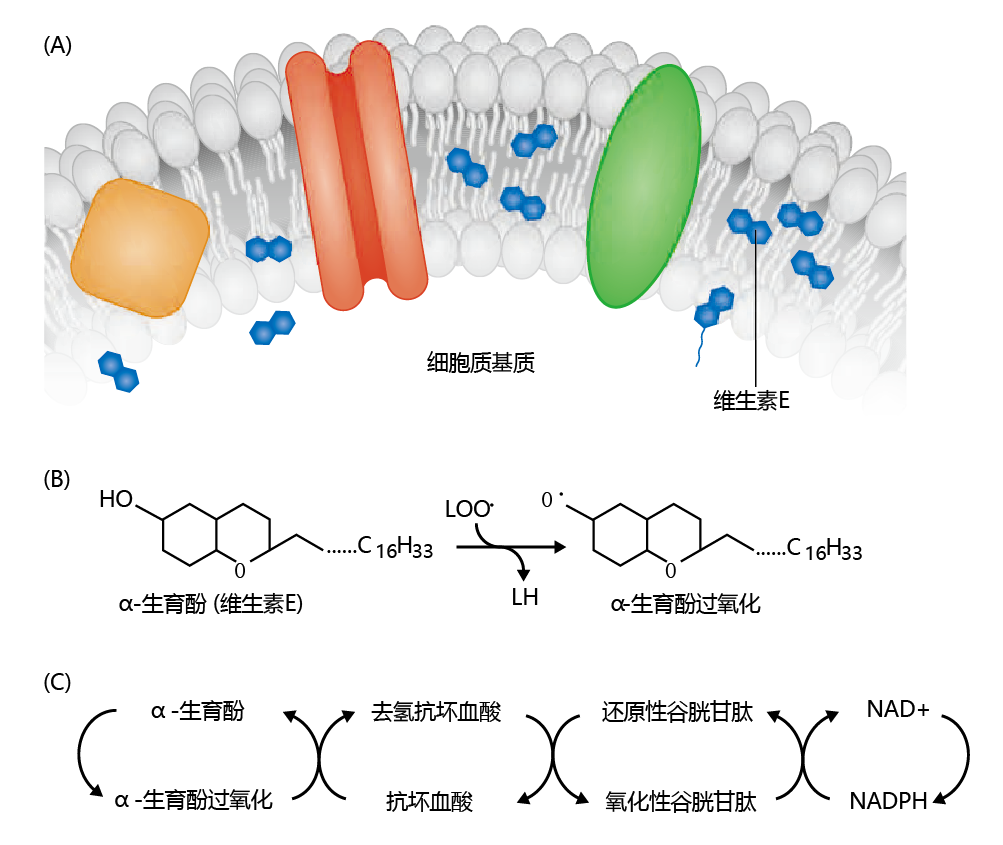
If the lipid-radical reaction that produces lipid peroxide was left unregulated, cellular function would be greatly hampered, if not altogether halted. Fortunately, the cell has developed a mechanism to prevent the formation of lipid peroxides, which involves vitamin E (tocopherol) and vitamin C (ascorbic acid). Vitamin E exists in the cell close to or within the cell membrane and has an affinity for the hydroxyl radical and the lipid radical that is considerably greater than that of the double bond of membrane polyunsaturated fats. As shown in Figure 4.29 , the lipid peroxyl radical is oxidized to LOOH by reduction of α-tocopherol to the α-tocopherol radical, thereby terminating the lipid peroxide chain reaction. However, the α-tocopherol radical needs to be oxidized back to reduced α-tocopherol. This is accomplished through a multi-step process that includes vitamin C (ascorbic acid), glutathione, and NAD+.
Figure 4.29 T ermination of the free-radical chain reaction by vitamin E (α-tocopherol). (A)Vitamin E is positioned in or near the cell membrane. (B)If a free radical arises, vitamin E is reduced to the α-tocopherol radical during oxidation of the lipid peroxyl radical. (C)The α-tocopherol is reoxidized in a multi-step process involving ascorbic acid, glutathione, and NAD+.
4.5.7 Reactive oxygen species can have beneficial effects
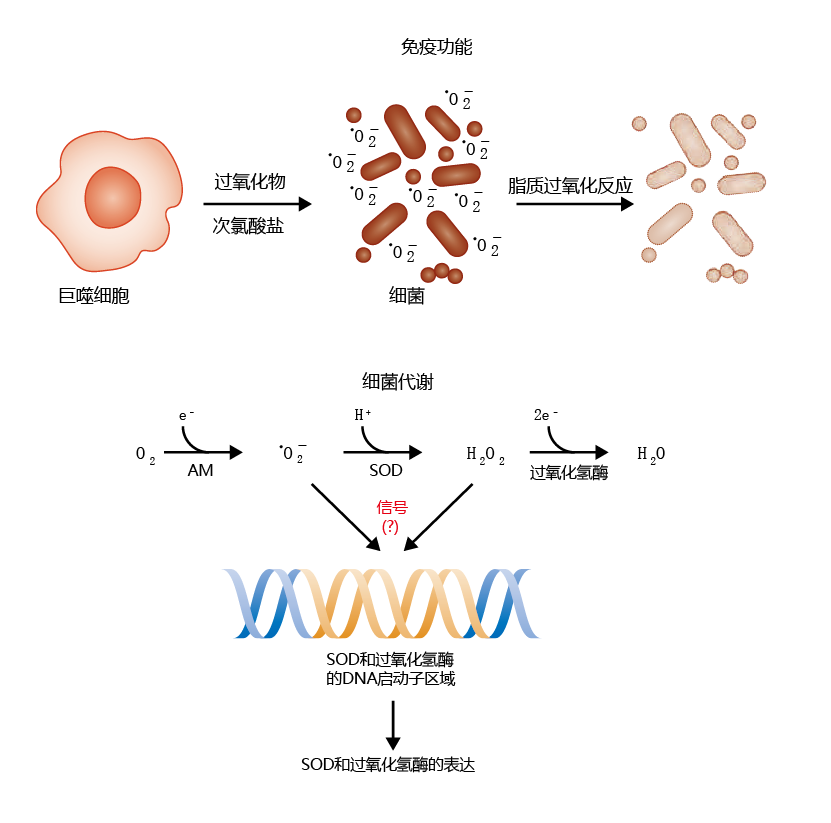
Our discussion so far has painted a fairly bleak picture of ROS. However, ROS can also have beneficial effects for the aerobic organism. The most widely know beneficial effect is found in the immune system. When a foreign invader, such as a bacterium, enters the bloodstream or tissues, the immune system releases a number of cells from the lymph nodes that attack and destroy the intruder. One of these cell types is the macrophages, which release enzymatically produced molecules designed to break down the cellular structure of the invading organism. Included among these molecules is • O2−, whose primary job appears to be breaking down the invader's cell membrane through the generation of the lipid peroxide moiety (Figure 4.30, top) . Recent research has also shown that ROS and/or by-products of ROS reduction, such as H2O2, may prevent oxidative damage by inducing further expression of the antioxidants superoxide dismutase and catalase (Figure 4.30, bottom). While the precise mechanism is still unclear, inducing production of the superoxide radical in cultured cells seems to activate the promoter regions of the genes for SOD and catalase.
Figure 4.30 Two examples of how ROS are beneficial to an organism. Immune function (top): Cells of the 免疫系统 called macrophages secrete toxic chemicals, such as the superoxide radical and hypochlorite, in response to an invading organism. The superoxide ions help to break down the cell membrane of the bacterium via the lipid peroxidation pathway. Aerobic metabolism (bottom): The generation of ROS during normal aerobic metabolism (AM) can also stimulate the expression of superoxide dismutase (SOD) and catalase, although the precise mechanism is unknown.