5.7 GENETIC REGULATION OF 寿命 IN M. MUSCULUS
The simple organisms S. cerevisiae and C. elegans have provided valuable information to support the possibility that reproduction, development, and longevity are linked, which in turn supports the 进化ary theories of senescence. These insights into the biology of aging and longevity have been further supported in studies of the significantly more complex Drosophila. Now we turn our attention to the common laboratory mouse. Mus musculus has a genome of 2.5 billion base pairs contained in 19 diploid autosomes and 2 diploid sex-linked chromosomes. The number of 蛋白质-coding genes currently stands at 21,839 and represents about 4% of the entire base-pair sequence.
The use of genetically modified mice has added significantly to our understanding of the genetic pathways that affect longevity. In general, the findings from longevity studies using genetically modified mice are consistent with the findings reported for S. cerevisiae, C. elegans, and Drosophila. That is, mice genetically altered to inhibit neuroendocrine pathways important to normal growth live significantly longer that the wild-type animals. This section explores the results of those investigations. First, we take a brief look at the challenges faced by biogerontologists in sorting out which genes affect longevity and which genes may have a greater role in slowing the rate of aging and delaying age-related disease.
5.7.1 Many M. musculus genes have been reported to affect 寿命
With the automation and commercialization of recombinant DNA technology, non-eneticists are able to construct knockout and transgenic mice with relative ease. Many of these genetically modified mice are being evaluated for their usefulness in biogerontological research. Between 30 and 40 different genes have been identified as having some impact on longevity in mice (TABLE 5.4). Several genetically modified mice have been developed to evaluate whether the longevity genes or the signaling pathways associated with these genes identified in S. cerevisiae, C. elegans, and D. melanogaster also extend longevity in a mammalian species. Other genetically modified mice have been developed to evaluate whether a gene known to have a role in a specific disease process also plays a role in differences in longevity.
TABLE 5.4 SOME GENE MUTATIONS IN M. MUSCULUS THAT EXTEND LIFE SPAN
In recent years, the virtual explosion of research evaluating the impact of a single gene on longevity in mice has provided significant advances in the field of biogerontology. It has also brought significant challenges in terms of interpretation and in determining the relevance of this research to the study of longevity. Many investigations suggesting that overexpression of a gene (transgenic strains) or elimination of a gene (knockouts) enhances longevity have failed to follow the standards for animal care and use procedures for biogerontology, as discussed in Chapter 2 (see Box 2.1). These failings include an insufficient number of animals for proper statistical analysis, studies performed on only one gender, insufficient details on housing conditions, and lack of pathology data. The absence of pathology data and probable cause of death may present the most significant challenge in determining the relevance of a gene in enhancing longevity. Since all the genes associated with increased longevity are also highly pleiotropic, it is possible that the overexpression or elimination of a gene has an effect on immunity and disease. For example, one study that lacked pathology data listed eight different phenotypic differences between the knockout mouse and the control. Without a description of pathology and/or probable cause of death, it is impossible to determine whether the gene affected longevity per se, a genetic effect, or whether the differences were due to an alteration in the rate of aging or prevention of disease, a stochastic effect.
Another challenge facing scientists trying to determine the relevance of longevity results from studies of genetically manipulated mice is the lack of replication of the findings. Repeating experiments to confirm the accuracy of the data is a cornerstone of the scientific method; without replication, results must be considered preliminary. In species that have a short life span—that is, S. cerevisiae, C. elegans, and Drosophila—hundreds of experiments on longevity can be completed within a couple of years, with minimal investment in housing and care of the organisms. In contrast, performing just two longevity experiments in mice would take nearly 10 years, at a cost of more than $130,000 for housing and care. The mean life span of the most commonly used strain of non-genetically altered mouse—the C57BL/6—is between 26 and 28 months; the maximum life span can be as much as 40 months. Genetically altering a gene of the C57BL/6 mouse has typically resulted in an increase in life span of about 25%. This means that the maximum life span may be as great as 50 months (4 years). In addition, the average cost of housing one mouse in a facility designed for longevity studies can be upward of $400. Consider a longevity investigation of mice, with 4 groups of 40 each (considered by most statisticians to be the minimum number needed for a significant longevity analysis); the cost of housing alone would be $64,000.
These methodological issues associated with many longevity investigations that use genetically manipulated mice do not lessen their importance to biogerontology. They simply make them less applicable to the study of longevity. Many transgenic and knockout strains have already provided significant insight into mechanisms underlying age-related disease. However, here we are concerned with the genetics of longevity, a process significantly different from that leading to age-related disease. For this reason, we discuss here only longevityenhancing genes that have been shown not to be affected by or to affect specific pathology and have been replicated, either in mice or in other species.
5.7.2 Decreased insulin signaling links retarded growth to 寿命
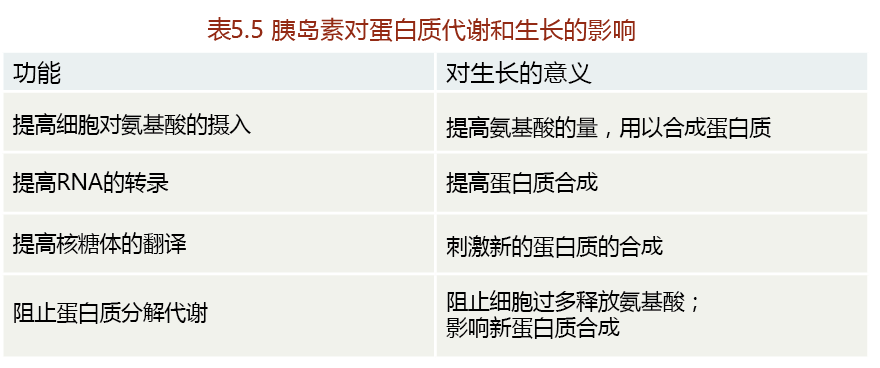
Recall that knockout mutations of C. elegans and Drosophila that target insulin/IGF-1-like signaling pathways retard development and/or growth and increase longevity. These results were important because insulin signaling pathways that affect growth are highly conserved phylogenetically and are found in both vertebrates and invertebrates (TABLE 5.5) . Therefore, it is likely that inhibition of insulin signaling in mice may also extend longevity.
TABLE 5.5 THE EFFECT OF INSULIN ON 蛋白质 METABOLISM AND GROWTH
Deleting genes that code for an intracellular signaling 蛋白质 and an insulin receptor increases longevity in mice, supporting similar findings in C. elegans and Drosophila. And like the knockouts in C. elegans and Drosophila, both of these genetically altered mice are considerably smaller than their wild-type counterparts. This once again suggests a close relationship between blunted growth and longevity. In one line of investigations, a gene encoding a major intracellular effector (an agent causing an effect) of insulin receptor binding called insulin receptor substrate 1 (Ins1) was eliminated. The increase in longevity observed in the knockout mouse was seen only in females. The gender difference in longevity of genetically altered mice is not an uncommon finding, the reason for which remains to be seen.
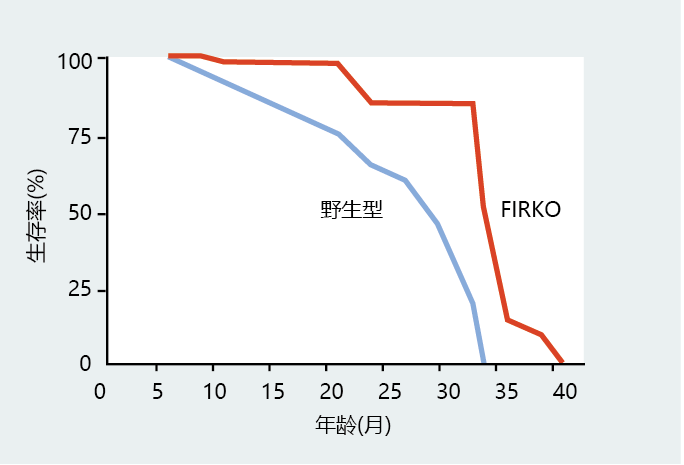
The other genetically altered mouse showing increased longevity lacks a gene that codes for an insulin receptor specific to adipose tissue, the fat-specific insulin receptor knockout, or FIRKO. The increased life span of the FIRKO mouse (Figure 5.33), although demonstrating once again the close relationship between insulin signaling pathways and longevity, also suggests that a reduction in fat storage may affect life span. In addition to its role in growth, insulin increases the amount of glucose that enters the fat cell. While only minuscule amounts of glucose are converted to fat, glucose provides the carbon backbone for the compound glycerol, a major component of triglycerides, the storage form of lipid found in adipose tissue. Since the adipose tissue in the FIRKO mutant lacks an insulin receptor, these mice have only limited amounts of adipose tissue, and the lack of adipose tissue explains their smaller size. You will learn in Chapters 9 and 10 that obesity has a major impact on mortality, and a healthy body weight reduces a person's risk for many age-related diseases.
Figure 5.33 Attenuation in insulin signaling pathways increases longevity. Survival curves for male and female mice, wild type and FIRKO (knockout mutant lacking the gene for a fat-specific insulin receptor). (Adapted from M. Bluher, B.B. Kahn, and C.R. Kahn, Science 299:572–574, 2003. With permission from AAAS.)
5.7.3 Diminished growth hormone signaling links insulin-like signaling pathways to increased寿命
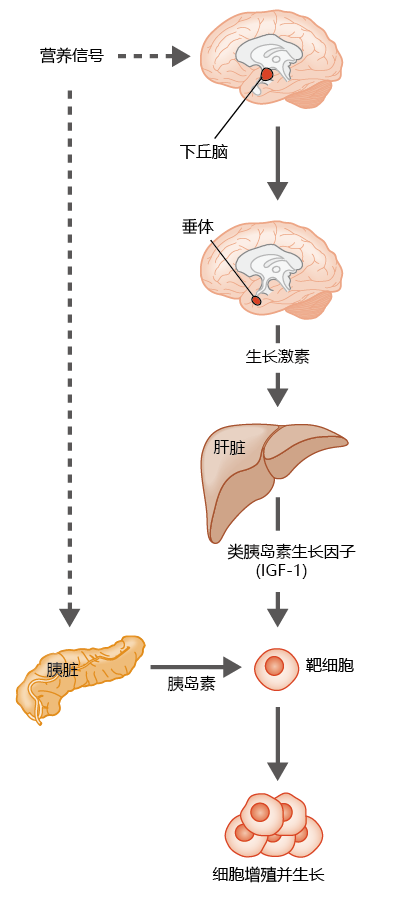
The reduction in body size caused by a disruption in insulin or insulin-like signaling pathways and leading to increased longevity is highly conserved in C. elegans, Drosophila, and M. musculus. Insulin and insulin-like signaling pathways in mammals are part of a much larger regulatory system that affects growth and development, involving the secretion of growth hormone from the pituitary gland (Figure 5.34). It is not surprising, therefore, that mutations causing a disruption in growth hormone signaling and thus stunted growth in mice result in increased longevity.
Figure 5.34 Simplified description of the growth hormone/insulin-like signaling pathway in mammals. The hypothalamus sends signals to the pituitary gland, located under the hypothalamus, causing secretion into the blood of growth hormone (somatotropin). Growth hormone stimulates the synthesis of insulin-like growth factor 1 (IGF-1) by the liver, and this factor is released into the general circulation. At the same time, the nutrient signal causes the pancreas to secrete insulin into the general circulation. Together, insulin and IGF-1 induce mitosis and growth of the organism.
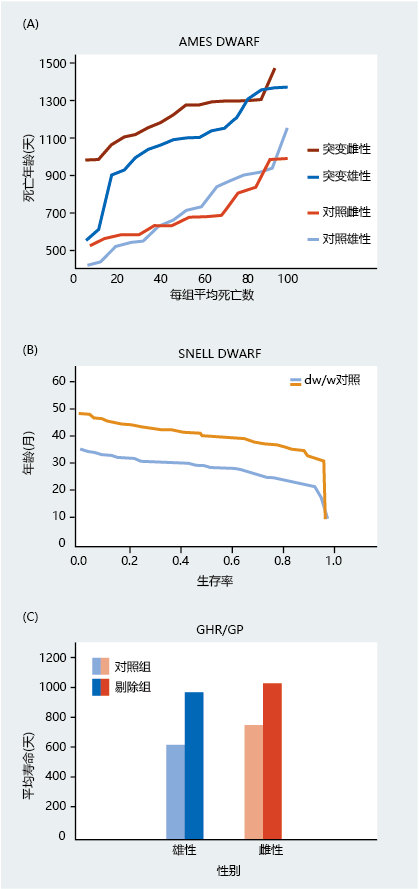
Three dwarf mutants—the Ames dwarf, the Snell dwarf (Pit-1), and GHR/GP (also known as GHRKO, growth hormone receptor knockout)—all have a disruption in growth hormone-initiated intracellular signaling that causes a smaller body size and increased longevity compared with the wild-type strain (Figures 5.35 and 5.36). The mutations in the Ames dwarf and the Snell dwarf are highly pleiotropic, resulting in a reduced blood concentration of growth hormone, prolactin, and thyroid hormone. The GHR/GP mutant does not show alteration in other hormones, but like the Ames dwarf and Snell dwarf, it has a reduced blood concentration of IGF-1.
Figure 5.35 GHR/GP knockout mice versus wild-type and heterozygous mice. Note that the GHR/GP knockout mouse (–/–) is smaller than its normal (+/+) and heterozygous (+/–) littermates. The difference in size among these three genotypes is similar to that seen in the other dwarf mouse strains, Ames and Snell. (From K.T. Coschigano et al., Endocrinology 141:2608–2613, 2000. With permission from the Endocrine Society.)
Figure 5.36 Longevity of three dwarf genotypes of the mouse. (A) Ames dwarf, (B) Snell dwarf, and (C) GHR/GP mutant. In the Ames dwarf and the GHR/GP mutant, females have significantly greater longevity than males. For the Snell dwarf, only males were evaluated. (A, adapted from H.M. Brown-Borg et al., Nature 384:33, 1996. With permission from Macmillan Publishers; B, adapted from K. Flurkey et al., Proc. Natl Acad. Sci. U.S.A. 98:6736–6741, 2001. With permission from PNAS; C, data from K.T. Coschigano et al., Endocrinology 141:2608–2613, 2000. With permission from the Endocrine Society.)
It also appears that the dwarf mutation slows the rate of aging and delays the onset of certain age-related diseases. For example, the time required for collagen to denature has proved to be an indication of the rate of aging in mice. This is because, once synthesized, collagen in the tail remains there for life and becomes more difficult to denature over time (the reason for this is explained in detail in Chapter 8). Thus, the faster collagen can be denatured, the biologically younger the 蛋白质 is. Collagen takes almost four times as long to denature in 19-month-old Pit-1 control mice as it does in mutants. In addition, while the incidence of neoplastic (cancer) diseases is virtually identical in the wild type and the dwarf mutant, the average age of appearance is significantly older in the mutant. Furthermore, for any given age, the dwarf mutant appears biologically younger. Although the mechanism underlying the life-extension properties that seem to delay age-related loss of function has yet to be identified, these mutations are providing biogerontologists with a method to examine longevity (genetic) and the rate of aging (stochastic) simultaneously.
5.7.4 Genetic regulation of 寿命 demonstrated in mice has implications for human aging
The increased longevity of genetically manipulated mice with an inhibited growth hormone/insulin-like signaling pathway is important because this demonstrates a phylogenetically conserved mechanism for longevity. Such consistency in results strengthens the 进化-based prediction that longevity is the by-product of genes selected for survival to reproductive age. The mouse data also confirm several other findings important to the study of biogerontology and human aging. For example, a mutation in a single gene can affect longevity in mammals. While the details of the mechanism underlying how the mutation increases longevity remain to be elucidated, the rate of aging in humans clearly can be evaluated at the gene level. In the years ahead, it is likely that research will identify drugs that target specific genetically regulated pathways, with the possibility of altering the rate of human aging.
The mouse data also confirm that increased longevity reflects neuroendocrine regulation, a finding that has major implications for humans. The success of humans as a species reflects, in part, our ability to survive and thrive in virtually every 环境. The ability to survive under harsh and sometimes stressful conditions is the result of a highly integrated system of neural pathways recognizing 环境al cues that induce secretion of hormones that maintain homeostasis—that is, the neuroendocrine system. For example, when children eat a meal, the nutrients are absorbed into the blood; the increased concentration of nutrients informs the brain that the building blocks of tissue are present and ready to be used; and the brain informs other tissues to secrete hormones that induce mitosis and the proliferation of tissue—that is, growth. You have seen how disruption in this pathway alters the rate of aging and longevity. It is a good bet that other neuroendocrine systems will have similar effects.
One final note on the genetic regulation of longevity. Although the highly conserved growth hormone/insulin-like signaling pathway has been the main focus of this chapter, it is not the only pathway that extends longevity. Its description in eukaryotes, from simple to complex, reflects the application of good scientific procedures in the study of biogerontological genetics. Other conserved pathways are certainly involved in longevity. To discover these pathways, research must be completed in precisely the same manner as that used in identifying the growth hormone/insulin-like signaling pathway. That is, begin by screening mutants that have enhanced longevity in short-lived species, then work up the phylogenetic tree to longer-lived mammals.
ESSENTIAL CONCEPTS
- Messenger RNA (mRNA) is the final product of transcription. When released from the nucleus into the cytosol, it contains only the nucleotides complementary to the DNA‘s exons, the coding portions of the gene.
- In the genetic code, three consecutive nucleotides, known as a codon, specify an amino acid.
- 蛋白质 are built one amino acid at a time. The ribosome recognizes that the 蛋白质 is complete when a stop codon (UAA, UAG, or UGA) is read on the mRNA.
- Histone acetylation, the binding of acetyl groups to certain amino acids on the histone tails, opens up the tightly pack histones so that the activator sites and promoter regions of the DNA are exposed. Histone deacetylation removes the acetyl groups and prevents gene expression.
- A gene can be cloned (identical copies made) in a quick, automated process called polymerase chain reaction (PCR). Once a gene has been cloned, its function can be predicted from the nucleotide sequence by applying the genetic code; this process has also been automated.
- Segments of a gene with unknown function can be labeled with fluorescent dyes and injected into cells, tissues, or whole organisms to determine where and when gene expression occurs. This method is called in situ hybridization.
- Evaluation of a gene‘s function in complex eukaryotes is commonly done by either removing the gene or inserting an extra copy of the gene. A mutant with the gene removed is called a knockout; a mutant with an extra copy of a gene is referred to as transgenic.
- Budding yeast dies after 20–30 cycles and can be used as a model for cellular replicative senescence. Budding yeast can also enter a stationary phase when 环境al conditions are not favorable from reproduction. This stationary phase can be used as a model for chronological aging.
- Gene silencing in yeast appears to be the primary mechanism that extends longevity. A few other gene-silencing pathways have been identified that are associated with low levels of food in the 环境.
- The roundworm C. elegans has a mechanism that prolongs development and delays reproduction. A larva in an environment that is not favorable to reproduction can develop into a dauer, which can survive in this 环境 for months. When 环境al conditions improve, the dauer re-enters the larval stage and develops into a reproductively active adult. • Biogerontologists have identified two genes in the dauer formation pathway, age-1 and daf-2, that also extend longevity. Both genes encode 蛋白质 that are highly conserved.
- Weak mutations in daf-2 extend life span but do not repress reproduction.
- Another C. elegans gene, the clock gene clk-1, also extends longevity. The pleiotropic nature of clk-1 has made identification of the underlying longevity-extending mechanism challenging. Because clk-1 regulates many functions in mitochondria, the most commonly accepted theory of the association between the gene and longevity is related to energy production.
- Several genes in Drosophila have been identified as influencing the rate of aging and longevity. In general, these genes code for 蛋白质 that have a role in protecting cells from stress that leads to cell damage and death.
- A long-lived Drosophila mutant, the Methuselah (mth) mutant, has extended longevity and exceptional stress resistance. Cloning of the mth gene revealed significant homology to a G-蛋白质-coupled receptor.
- A mutant strain of Drosophila known as chico contains a lossof-function mutation (knockout) that reduces the expression of an insulin receptor substrate, CHICO. The chico gene is highly homologous to the daf-2 mutant described in C. elegans.
- The extended longevity of the chico fly is associated with growth arrest through a neuroendocrine mechanism. A deficiency in juvenile hormone, which is needed for proper growth and development, has been shown to be a phenotype of the chico fly.
- Inhibition of insulin-like signaling that leads to increased longevity has been demonstrated in M. musculus. The enhanced longevity of these mutants reflects stunted growth caused by a disruption in the growth hormone/insulin-like signaling pathway.
- The conservation of genes that affect longevity, from simple to complex eukaryotes, has several implications for human aging, including (1) a mutation in a single gene can influence longevity; (2) longevity reflects, at least in part, neuroendocrine regulation; and (3) other genetic pathways associated with longevity must exist and await elucidation.
本章结束