5.6 GENETIC REGULATION OF 寿命 IN D. MELANOGASTER
The mutation of individual genes in simple organisms such as S. cerevisiae and C. elegans has shown that longevity can have a highly regulated genetic component. The use of these simple organisms is aided by the fact that most of their genes have a single function and are expressed in response to a single biochemical pathway or 环境al stimulus. That is, the gene regulatory pathway resulting in an alteration in life span can be isolated rather easily in these simple organisms, relative to more complex life forms. As we move up the 进化ary ladder, the levels of anatomical and physiological complexity are the result of an increased level of regulation of gene expression. Genes in complex organisms tend to be highly pleiotropic—they affect more than one physiological function—and the expression of any one gene can be regulated by several pathways. Thus, isolating and describing pathways of the genetic regulation of longevity tends to be more difficult in complex organisms than in simple organisms such as S. cerevisiae or C. elegans.
The description of genetic regulation of longevity in complex organisms such as D. melanogaster has been limited, for the most part, to lifespan analysis in genetically selected strains. These results have clearly shown that mutations in many different gene loci can extend longevity (TABLE 5.3). Such results, while supporting the possibility of genetic regulation of longevity, cannot conclusively demonstrate that a specific gene encoding a specific 蛋白质 is directly responsible for extending life span. Given our new understanding of how genes are regulated (see the earlier section “Regulation of Gene Expression”), it is probable that the mutated gene that imparts alterations in longevity may be just one part of a more complex pathway. Therefore, to minimize unnecessary speculation and uncertainty with regard to our discussion of the effects of genes on life span, our exploration into the genetic regulation of longevity in complex organisms focuses primarily on pathways that appear to be highly conserved, from yeast to human. As you‘ll see, these pathways have a connection to neurohormonal signaling, similar to that found in experiments with C. elegans. We begin with a brief exploration of Drosophila mutants that have extended longevity, to set the stage for a more in-depth analysis. Then we discuss three pathways that demonstrate, in Drosophila, the connection between neurohormonal signaling and longevity.
TABLE 5.3 SOME GENE MUTATIONS IN DROSOPHILA THAT EXTEND LIFE SPAN
5.6.1 Drosophila has a long history in genetic research
Most of what we know about classical and molecular genetics has come from studies of Drosophila melanogaster. The first successful breeding of a Drosophila for a specific trait was reported more than 100 years ago. Using Drosophila, Thomas Hunt Morgan (1866–1945) showed that genes are contained within the chromosome, for which he won the Noble Prize for Medicine in 1933, the first ever for a geneticist. The discovery that the genes regulating the placement of body parts, the Hox genes (see Box 5.1), are virtually identical in all multicellular organisms came, in large part, from studies of Drosophila.
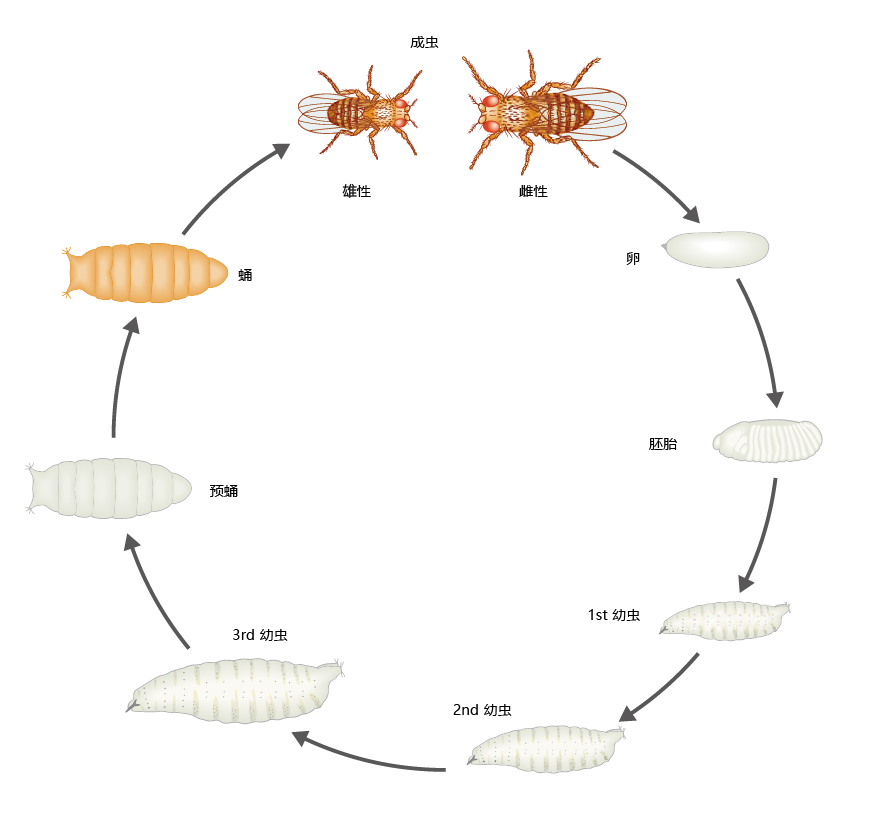
The genome of Drosophila contains approximately 165 million base pairs, of which about 20% make up 14,000 genes. The DNA is contained in four diploid chromosomes, three autosomes and one sex chromosome. The Drosophila life cycle consists of six stages: embryogenesis, three larval stages, a pupal stage, and an adult stage (Figure 5.27). Development time, from egg to adult, takes approximately nine days under ideal temperature conditions (25°C) and with sufficient food, but can take longer if temperature and food are less than optimal. Males are smaller than females. Females are receptive 12 hours after emergence and can remain fertile for 15–25 days. The average life span of wild-type Drosophila is 40–50 days.
Figure 5.27 Stages of the Drosophila life cycle.
5.6.2 Genes that extend 寿命 are associated with increased stress resistance
Table 5.3 lists some genes that have been identified as extending longevity in Drosophila. Many of these genes have a common function, and that function is anti-stress. For example, the chaperone 蛋白质 encoded by hsp70 (heat shock 蛋白质) belongs to a group of 蛋白质 that mark misfolded or damaged 蛋白质 for degradation through the ubiquitin pathway (see “蛋白质 Can Be Modified or Degraded after RNA Translation,” earlier in this chapter). Extensive work has been completed in Drosophila and mice to show that overexpression of hsp70 can lead to extended longevity. Another group of 蛋白质 involved in anti-stress functions and repeatedly shown to have a significant influence on the rate of aging and longevity in Drosophila is the endogenous antioxidants, 蛋白质 that degrade oxygen-centered free radicals. Overexpression of superoxide dismutase, one enzyme in the pathway that reduces the superoxide radical to water, has consistently, but not always, extended longevity in Drosophila. While it is clear that heat shock 蛋白质 and endogenous antioxidants are involved in extended longevity in Drosophila, they are expressed in response to other intracellular signals. That is, some gene(s) upstream from the genes for these two 蛋白质 must be the factor that accounts for the extended longevity. Those genes await identification.
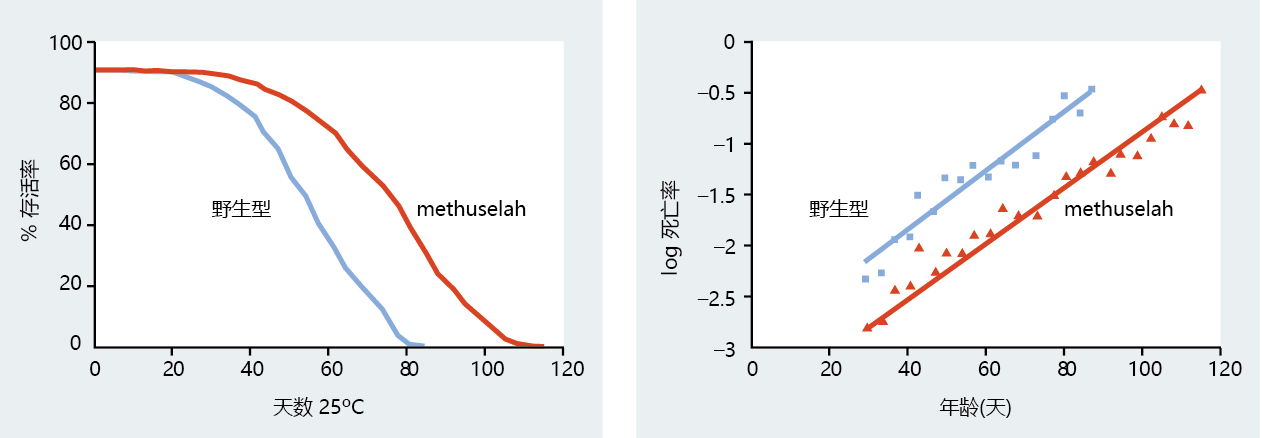
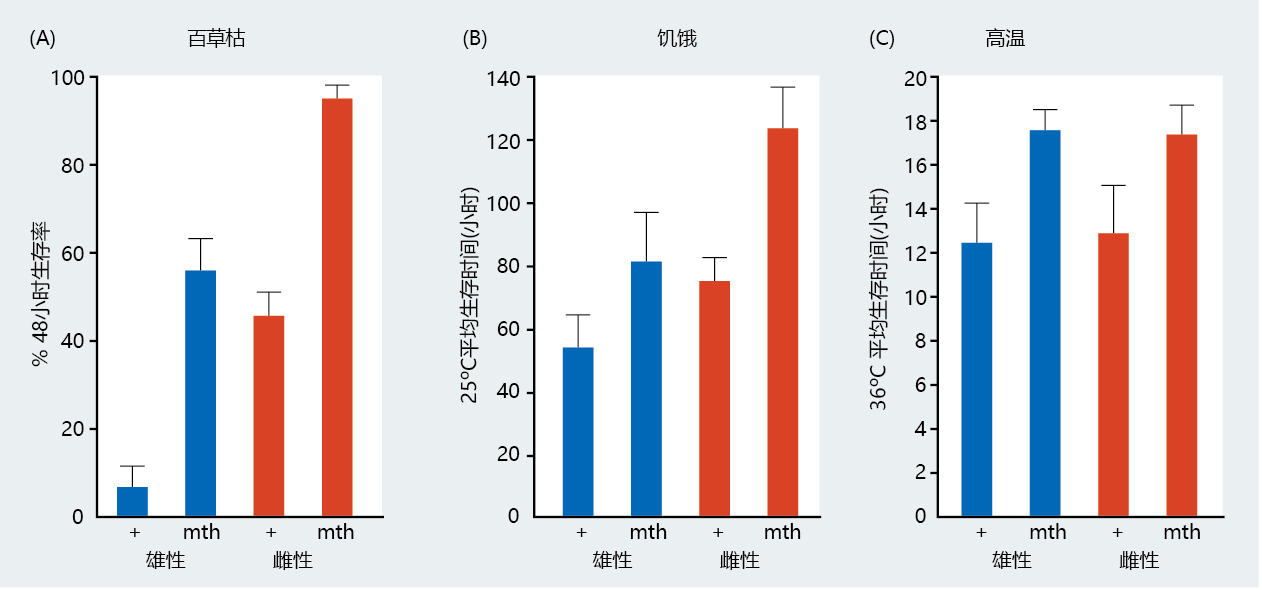
The connection between anti-stress genes and longevity became clearer with the discovery and cloning of the Methuselah (mth) gene. (Methuselah is a biblical character who was reported to live 969 years.) Careful screening of Drosophila mutants identified a long-lived strain that was heat resistant. The transgenic mth fly was shown to live significantly longer than the wild type (Figure 5.28), and it was more effective at resisting the stress of paraquat (a chemical that induces overproduction of oxygen-centered radicals), starvation, and high temperatures (Figure 5.29). Cloning of the mth gene revealed significant homology to a G-蛋白质-coupled receptor. Recall that, in yeast, a G-coupled receptor has been identified with extended longevity. The central role of G-coupled receptors in longevity was confirmed when the binding of ligands specific to G-coupled receptors extended the life span of the transgenic mth Drosophila mutant.
Figure 5.28 Survival curve and log of mortality rate for wild-type and mth transgenic Drosophila. Note that the log of mortality for the mth transgenic strain is shifted to the right, indicating that the rate of aging has been slowed. (From Y.J. Lin, L. Seroude, and S. Benzer, Science 282:943–946, 1998. With permission from AAAS.)
Figure 5.29 Survival of Drosophila in response to stress. These graphs show the survival of male and female wild-type (+) and mth transgenic Drosophila in response to (A) paraquat, (B) starvation, and (C) high temperature. In all treatments, male and female flies carrying the mth mutation survived longer than the control, wild-type flies. (From Y. J. Lin, L. Seroude, and S. Benzer, Science 282:943-946, 1998. With permission from AAAS.
5.6.3 Genes controlling Drosophila‘s growth also extend life span
You have learned that extended longevity in S. cerevisiae and C. elegans appears to be tightly coupled to 环境al conditions, which, in turn, affect reproduction. 环境al conditions that place stress on the animal, such as scarcity of food, repress gene expression and halt growth and reproduction until the 环境 is more favorable to the survival of offspring. The signaling pathways that repress growth and reproduction in S. cerevisiae and C. elegans also extend longevity. You have also learned from experiments in C. elegans that the signal initiating dauer formation and extended longevity is of neuroendocrine origin. Drosophila, like many insects, has also developed a strategy for delaying reproduction during times of poor 环境al conditions. This strategy, called diapause, seems to be heavily influenced by neurohormonal signals and is characterized by reproductive silence, reduced energy metabolism, and resistance to stress. Biogerontologists have taken advantage of diapause in Drosophila to evaluate genes that affect longevity.
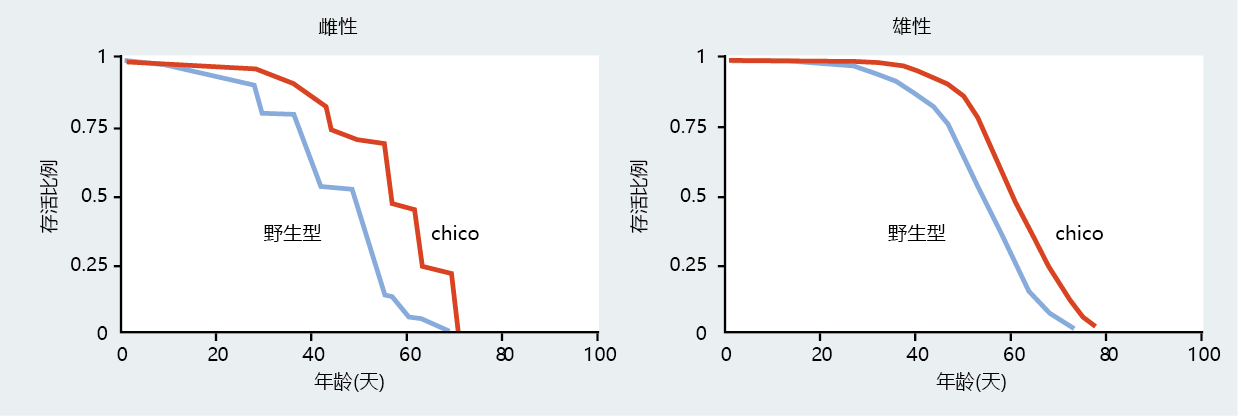
A mutant strain of Drosophila known as chico (Spanish for “small boy”) has provided further evidence to suggest that insulin-linked gene regulatory pathways, those associated with growth and reproduction, may play a significant role in the rate of aging in species more complex than C. elegans. The chico flies are about half the size of wild-type Drosophila; the size difference is the direct result of this mutant having fewer and smaller cells (Figure 5.30). The chico mutant contains a loss-of-function mutation (knockout) that reduces the expression of an insulin receptor substrate, CHICO. The chico gene is highly homologous to the daf-2 mutant of C. elegans, and the reduced expression of chico has been shown to extend mean life span in Drosophila (Figure 5.31). Moreover, the CHICO 蛋白质 stimulates growth through a pathway similar to that described in C. elegans: the inhibition of a forkhead transcription factor, dFOXO.
Figure 5.30 Female chico mutant (left) and wild-type (right) adult Drosophila. (From M.D. Piper et al., J. Intern. Med. 263:179 –191, 2008. With permission from Wiley.)
Figure 5.31 Life span of female and male flies with and without the chico gene. Increased survival of the chico knockout was limited to mean life span. This may indicate that chico plays a larger role in slowing the rate of aging than in directly increasing longevity. (From D.J. Clancy et al., Science 292:104–106, 2001. With permission from AAAS.)
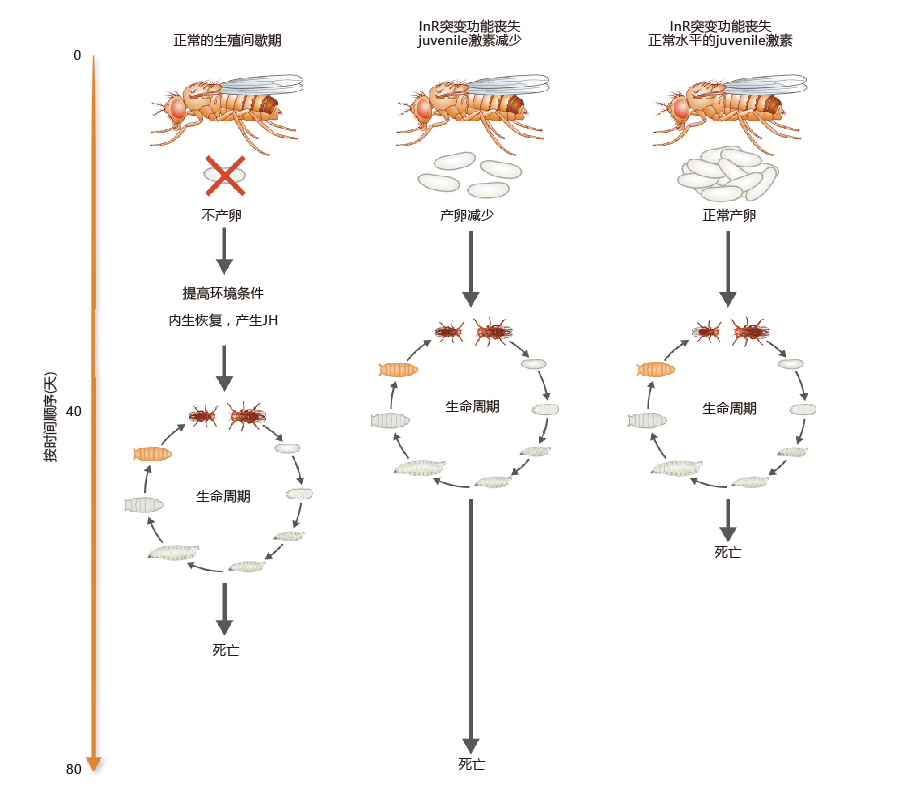
The possibility that an 进化arily conserved mechanism involving an insulin pathway may participate in the regulation of life span gained further support with the finding that a loss-of-function mutation in an insulin pathway receptor, InR, also increased life span. This mutation resulted in a significant decrease in kinase-like activity. A unique characteristic of this loss-of-function mutant is that egg production decreases significantly compared with wild-type flies, a finding linking this mutation to diapause (Figure 5.32) . The link between reproductive diapause and longevity grew even stronger when it was found that administration of a hormone involved in larval development, juvenile hormone (JH), restores the function of InR in the adult fly and decreases life span.
Figure 5.32 Relationship between reproductive diapause, juvenile hormone (JH), and life span. Drosophila in reproductive diapause does not lay eggs. Egg laying resumes when 环境al conditions improve, and life span is extended relative to the wild-type fly. Loss-of-function mutation in an insulin-like receptor (InR) reduces egg production and extends life span (center). Treating the InR-mutated flies with JH can restore normal egg production (right), but this treatment decreases life span.