7.1 人类寿命起源
Recall from Chapter 3 that results from laboratory experiments using artificial selection in Drosophila were consistent with the 进化 theory of longevity proposed by Peter Medawar and with the mathematics of W. D. Hamilton. Similar laboratory experiments cannot be done in humans. Therefore, we must look to other, noninvasive methods for confirmation of the 进化ary theory of longevity. The emerging field of biodemography—the science that integrates biology and demography—provides the methods to investigate the origins of longevity that are uniquely human. Biodemography is primarily a mathematical and theoretical science that constructs models to predict the origins of human longevity. These mathematical models integrate the principles of mortality analysis with experimental observations from scientific fields such as archeology, physical anthropology, genetics, and 进化.
In this section, then, we explore the origins of human longevity through the science of biodemography. We start with a few general biodemographic principles that guide the development of models predicting the origins of human longevity, and these principles lead us to an emerging theory suggesting that the origins of longevity in humans may be unique among species, reflecting our superior intelligence and our ability to manipulate the 环境.
7.1.1 Human mortality rates are facultative
Molecular and cellular observations made in simple eukaryotes and rodents have allowed us to separate, with a fair amount of precision, the differences among aging, longevity, and age-related disease. Results from highly controlled laboratory experimentation in animals have led to a definition of longevity as a by-product of genes selected for survival to reproductive age (see Chapter 3). Using similar laboratory techniques, researchers have observed that another measure of the length of life, the mean life span, seems to be more closely related to random, or stochastic, effects occurring as a matter of chance during development. As a result, we have been able to define longevity as the maximum age potential of a particular species, and life span as the length of life of an individual within the species.
The study of human mortality in the discipline of biodemography cannot easily separate the genetic, intrinsic rate of mortality from the environmentally dependent, extrinsic rate of mortality (see Chapter 2 for a discussion of “intrinsic” and “extrinsic” rates in the context of aging). Nor should environmental factors that affect human longevity be isolated. Human deaths due to age-related disease or environmental factors are accounted for mathematically in biodemographic predictions concerning the origins of human longevity. That is, biodemographers view environmental factors as critical variables in their mathematical models. Humans respond to environmental changes through cognitive reasoning, rather than simply reacting with pure instinct. Humans can change the environment to fit their needs, and this manipulation has resulted in mortality and longevity characteristics that are unique to H. sapiens.
The human ability to manipulate the environment reflects one of the most important principles of biodemography: mortality rates are facultative. The term facultative, as used in biodemography, means that environmental influences cause the mortality rate, or the trajectories of the mortality rate, to have significant plasticity. That is, the rates are not fixed, like those predicted by Gompertz analysis (see Chapter 2). This plasticity arises from the fact that the differences in environmental conditions that influence the mortality of individuals in human populations are infinite and are continually changing. Although the overall population mortality rate may take on a particular Gompertz mortality pattern, the rates for subpopulations will differ significantly. Many biodemographers believe that these subpopulations have had a significant influence on the origins of human longevity.
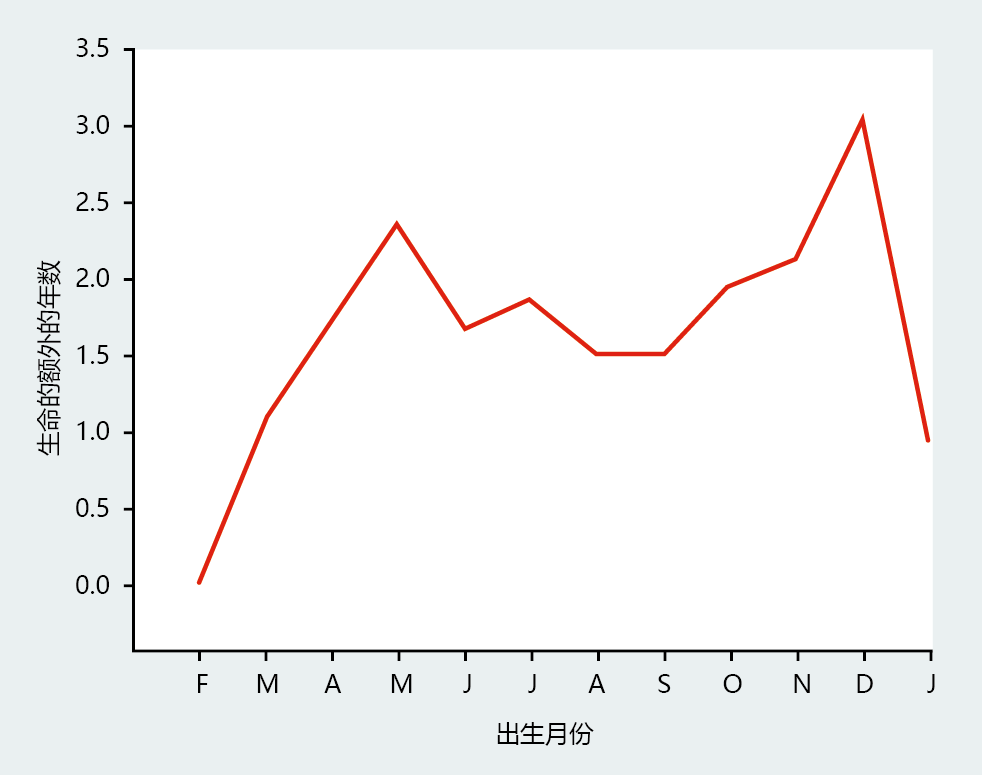
We can use the example of the seasonal variation in births in a historical population to demonstrate the facultative nature of mortality and its effect on longevity (Figure 7.1). Babies born during periods in which the quantity and quality of nutrition are greatest—that is, the months during and immediately after the harvest (in the Northern Hemisphere, September–December)—have longer lives than those born in the winter months, when food shortages are most likely to occur (January and February). This simple correlation suggests that environmental factors during early life have established multiple subpopulations with distinctive rates of mortality. Because infant mortality would be lower for babies born during the harvest months, these subpopulations would have greater fitness, with a greater impact on life span and/or longevity.
Figure 7.1 A biodemographic prediction of additional years added to the human female life span as a function of birth month. The research suggests that differences in longevity may reflect differences in nutritional factors at the time of birth. These results were generated using regression analysis of the length of life of 6908 women over the age of 30 born in European countries between 1800 and 1880. (From L.A. Gavrilov and N.S. Gavrilov, in Modulating Aging and Longevity [S.L.S. Rattan, ed.], Dordrecht, Netherlands: Kluwer Academic Publishers, 2003. With permission from Springer Science.)
7.1.2 Genetic factors cause signifi cant plasticity in human mortality rates
Human mortality is highly variable, and this variability remains even after controlling mathematically or statistically for the confounding factors of age-elated disease and environmental influences. Moreover, biodemographic research has shown that included within the overall human mortality rate are discrete subpopulations of individuals sharing a specific Gompertz mortality rate that differs from that of the population as a whole. Together, the non-environmental variability and the existence of discrete subpopulations with their own mortality rates strongly suggest that genetic factors influencing human longevity also have plasticity and are not fixed. (Experimental evidence for the genetic plasticity of mortality rates was discussed in Chapter 2.) Studies in the Mediterranean fruit fly demonstrate that late-life mortality departs from the Gompertz rate by leveling off and then decelerating (see Figure 2.19). This late-life difference in mortality rates is generally accepted to mean that different genotypes for longevity exist within populations. Biodemographers suggest that these different genotypes could have existed throughout evolutionary history and may have played a role in the selection of genes that determine human longevity.
The effect of genetic plasticity on mortality rates provides biodemographers with an evolutionary basis for predicting the origins of longevity in humans. Imagine a population of early hominids having enough genetic plasticity to produce different mortality rates. This population would contain a subpopulation of individuals that died before reaching the end of their reproductive life span and, at the other extreme, a subpopulation that lived well beyond the end of their reproductive life span. The longer reproductive life span of the long-lived subpopulation would endow greater fitness, albeit only slightly greater than for the shorter-lived subpopulation. Over evolutionary time, genes producing the genotype of the longer-lived, longer-reproductive-period subpopulation would be selected over genes producing the genotype of the shorter-lived population. (See the discussion of genetic drift and mutation accumulation in Chapter 3.) The human genome would have drifted, and would still be drifting, toward increased longevity.
7.1.3 Mortality rates differ in long-lived humans
In the past, testing the possibility that genetic factors account for differences in the mortality rates of the longest-lived humans has been difficult, simply because not enough individuals reached an advanced age. Evidence for differences in mortality rates was generally limited to observations of families with several individuals who reached an advanced age. Today, however, several scientific studies are being conducted, throughout the world, that explore the relationship between genetics and life span in cohorts of long-lived humans, normally defined as people over the age of 100—centenarians. (In the United States, the number of people over the age of 100 is somewhere between 100,00 and 200,000.) These investigations have only just begun, and the analysis is limited, for the most part, to demographic studies. While some studies of centenarians are beginning to identify specific alleles associated with extreme longevity, these results are far too preliminary for discussion here.
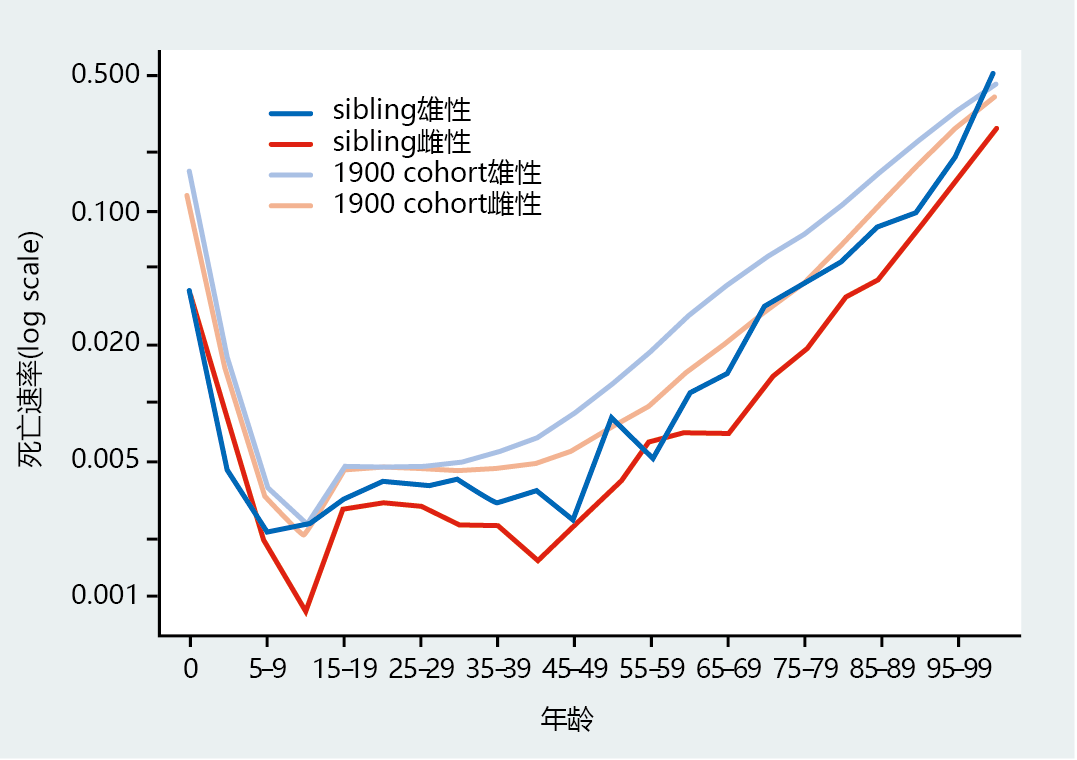
One approach to investigating the genetic contribution to human longevity is to evaluate the mortality rates of centenarians' siblings or twins. Siblings or twins of centenarians are highly likely to have a similar genetic profile, providing demographic evidence that longevity may be related to genetics. Several studies have taken this approach and have shown similar results: the survival probabilities and mortality rates of the siblings or twins of centenarians differ significantly from the mortality rate in the general population. For example, the New England Centenarian Study found that female and male siblings of centenarians were, respectively, 8 and 17 times more likely than the general population to reach the age of 100. This same study showed that, at all ages throughout the life span, centenarians‘ siblings had mortality rates that were roughly half of those observed for the United States as a whole (Figure 7.2) . While much research is still needed to confirm the genetic component of human longevity, it appears that genes have a significant role in determining longevity.
Figure 7.2 Age-specific death rates by gender in a 1900 birth cohort and in siblings of centenarians. These data show that age-specific death rates are significantly lower at all ages for the siblings of centenarians than for the general US cohort born in 1900. (See Figure 2.15 for an explanation of the change in slope of age-specific mortality.) (From T.T. Perls et al., Proc. Natl Acad. Sci. U.S.A. 99:8442 –8447, 2002. With permission from National Academy of Sciences.)
7.1.4 Human intelligence has altered mortality rates
Darwin‘s principle of reproductive fitness closely links fitness to a species‘ adaptation to environmental conditions. For the majority of species on Earth, adaptation occurs purely as a chance event, when an allele arises in an individual that increases the probability of its survival to reproductive age. That is, for most species, mortality rates reflect an adaptation to the environment (see Chapter 1). The origin of longevity in Homo sapiens may have resulted from our advanced intelligence, which has allowed us to adapt the environment to our genes and thus change mortality characteristics. For example, the use of tools, such as sharpened stones and bones, enhanced our ability to hunt and increased the variety of foods in our diet. Early H. sapiens would have had significantly better nutrition, which contributed to our larger size compared with other primates—a significant survival advantage. Invention of the needle and thread led to better clothing, which allowed us to inhabit geographical locations different from those in which our physiology had evolved. We were no longer dependent on the local environment to provide food; we could move to where the food was and thus gain a significant survival advantage.
The intelligence that allowed H. sapiens to manipulate the environment for a survival advantage would probably have had a significant impact on the survival of mothers and infants. The increase in the quality and quantity of food and nutrition, along with advances in simple technology (tools), would have reduced maternal deaths at birth—which, most likely, would have had two outcomes that decreased mortality rates and increased longevity.
First, mothers surviving birth would live to have more children. Having more children increased the chance of at least one child, if not more, surviving to reproductive age. Surviving to reproductive age meant the individual had superior genes, better at fending off infections and other detrimental environmental conditions. Thus, what arose as a product of intelligence—reduced maternal deaths at birth—may have resulted in children with genes imparting lower mortality rates and greater longevity.
Second, better nutrition and better protection from the elements would have allowed the investment of more resources in offspring and thus improved the infant mortality rate. As you‘ll see in the next section, infant mortality rates have a great impact on life span. A decreased infant mortality rate might also have led to fewer births, allowing parents to concentrate their resources on fewer children and to improve the overall quality of their offspring.
7.1.5 Human intelligence has produced a unique 寿命 trajectory
The uniquely human ability to manipulate the environment, due to our superior intelligence, has been fundamental in shaping the characteristics of human longevity. However, researchers cannot directly measure intelligence in our phylogenetic predecessors—one cannot give intelligence tests to fossils. Instead, biodemographers often turn to the indirect measures of intelligence used in physical anthropology and evolution/ecology to support their mathematical predictions on the origins of longevity in H. sapiens. To this end, brain size, measured directly in living animals or deduced from the size of the brain cavity of fossilized skulls, has been used to approximate intelligence.
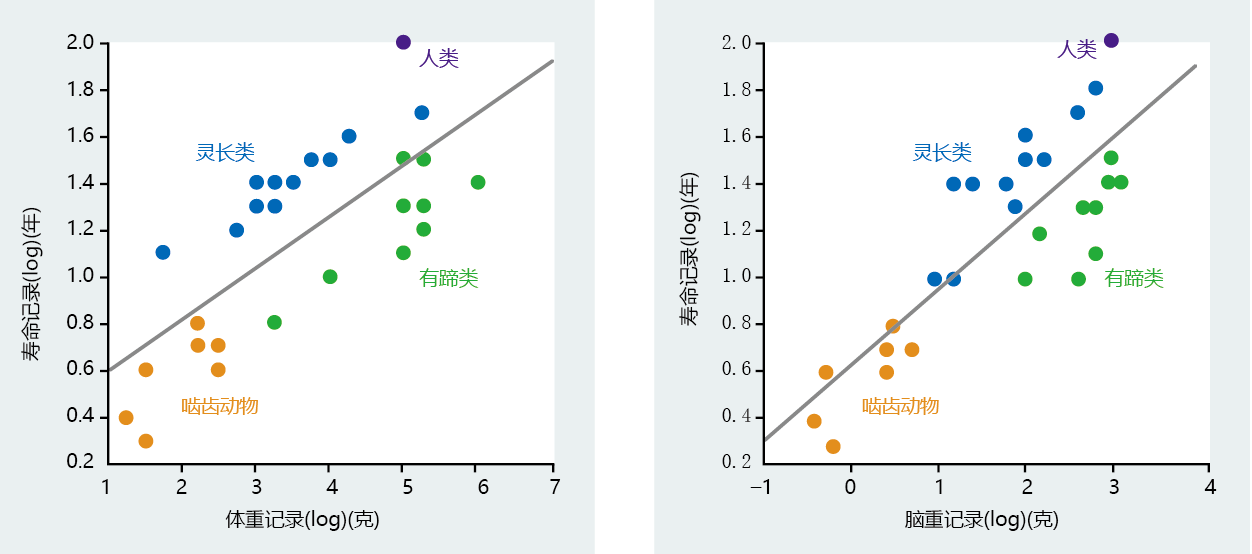
The positive correlation between brain size, body weight, and longevity in mammalian species has been known for several decades (Figure 7.3) (see also Chapter 1). In general, the larger the body and brain weight, the longer the life span. These early correlations also describe a distinctive phylogenetic separation between primates and non-primates. Note in Figure 7.3 that the data points for primates and humans fall above the regression line, whereas the data points for rodents and ungulates fall below the regression line. This means that the body/brain weight-life span correlation in primates (human and nonhuman) is separate from that in other phylogenetic groups. Moreover, the body/brain weight–life span correlation in humans is significantly different from that in other primates, suggesting yet another phylogenetic separation for intelligence among primates. For these reasons, biodemographers generally limit their analysis of the origins of human longevity to the primate order.
Figure 7.3 Correlation of body weight and brain weight with life span in various mammalian groups. Note that the human life span is considerably greater than that of ungulates (hoofed animals, in several orders of mammals) of similar body weight. A significantly larger brain underlies the difference in life span between humans and the other three groups of mammals. (From G.A. Sacher, in Lifespan of Animals [G.E.W. Wolstenholme and M. O‘Connor, eds.], London: J.A. Churchill, pp. 115–141, 1959. Little Brown and Co. With permission from Elsevier.)
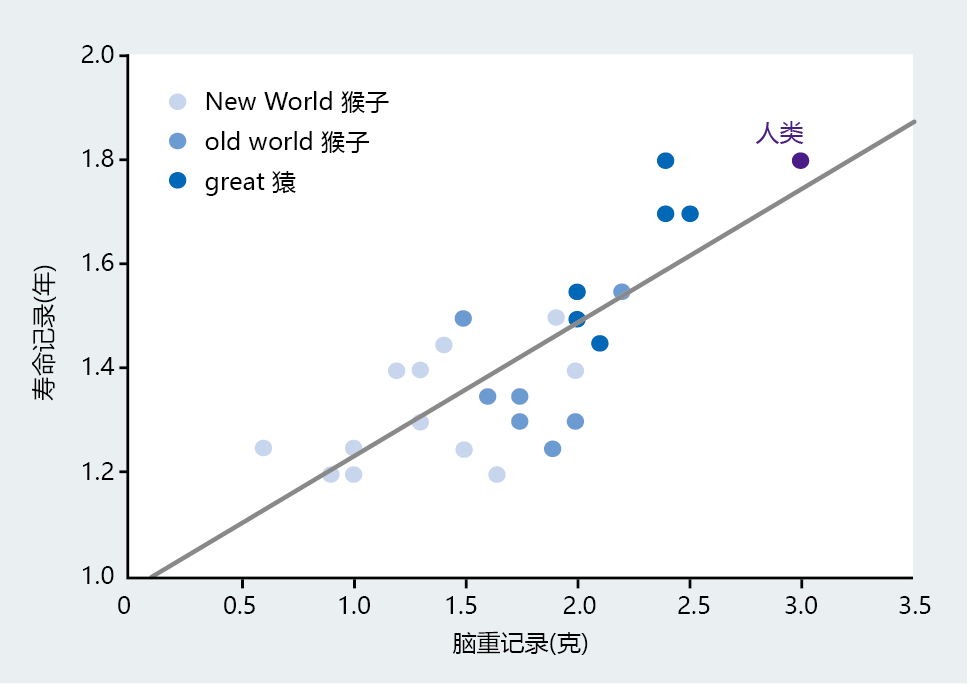
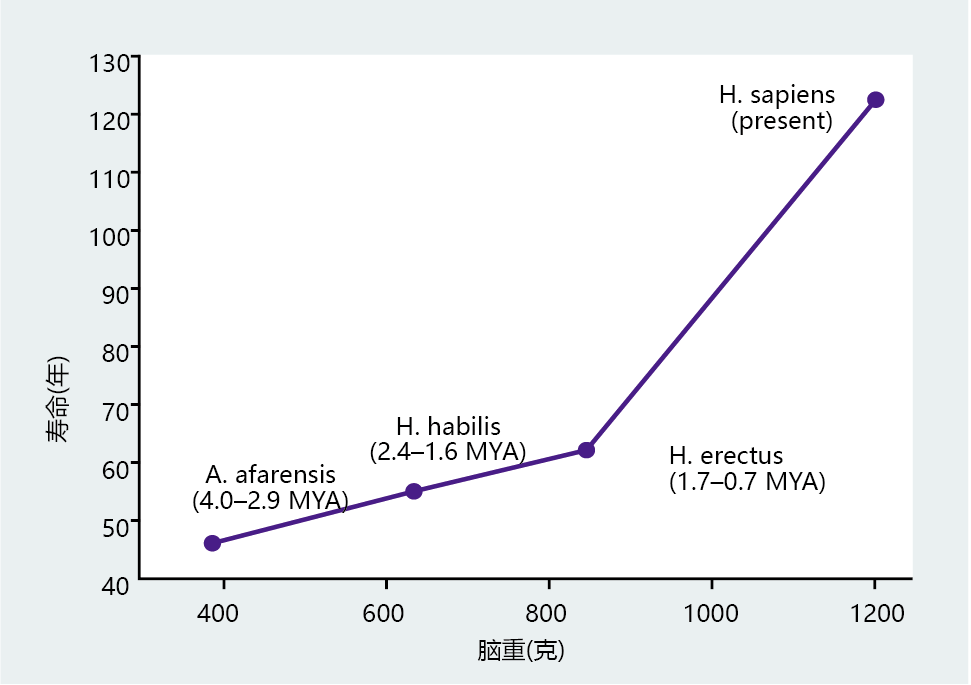
New World and Old World monkeys have significantly smaller brains and shorter life spans than humans (Figure 7.4). Moreover, the great apes with body weights similar to that of humans have smaller brains and a shorter life span. If we narrow the analysis even further by considering only hominids, the strong relation between brain size and life span remains (Figure 7.5). There is also a tremendous shift in life span between our immediate phylogenetic relatives and modern humans. The life span difference between Australopithecus afarensis and Homo erectus is approximately 15 years, with a change in brain weight of 450 grams taking place over 3 million years. H. sapiens developed a brain weight almost 400 grams greater than that of H. erectus in less than 700,000 years, which translated into a gain in life span of 60–70 years. Thus, the anthropological data indicate that intelligence, as measured by cranial volume, set H. sapiens on a mortality and life-span trajectory distinctly different from that of our immediate phylogenetic predecessors.
Figure 7.4 Correlation between brain weight and life span in humans and their immediate phylogenetic relatives. These data indicate that brain weight, even among morphologically similar species, has been a critical factor in the origins of human longevity. (From J.R. Carey, Longevity: The Biology and Demography of Lifespan, Princeton, NJ: Princeton University Press, 2003. With permission from Princeton University Press.)
Figure 7.5 Correlation between brain weight and life span in humans and earlier hominids. Life spans of Australopithecus afarensis, Homo habilis, and Homo erectus were estimated from fossils on the basis of dental and bone development. Brain weights in these hominids were estimated from brain-cavity volume. MYA = million years ago. (From H.M. McHenry, J. Hum. Evol. 27:77–87, 1994. With permission from Elsevier.)
7.1.6 Heredity has only a minor infl uence on human life span
We have stressed that intelligence allowed early Homo species to manipulate their environment, thus increasing survival and establishing conditions that could have resulted in a genetic drift toward greater longevity. In other words, human longevity seems to have followed the prediction of the evolutionary theories proposed by Medawar and Hamilton that you learned about in Chapter 3. The term “life span,”
as you‘ll recall, applies to individuals within a species rather than to the species itself and is determined largely by an individual's rate of aging. That is, an individual‘s life span is determined by random events occurring over time. So, if the evolutionary theories are valid, we would expect heredity to have a minor effect on life span.
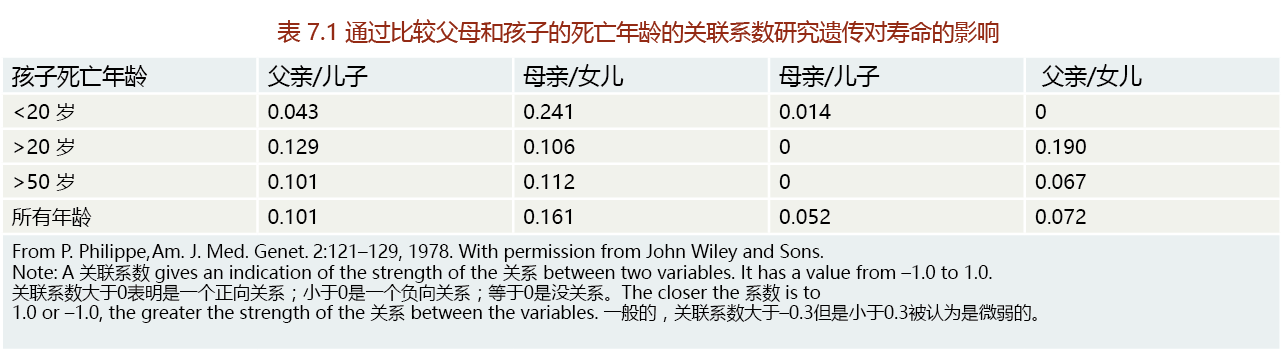
We can test this possibility by evaluating the correlation between the age of death in parents and their children and in twins. Weak correlations would suggest that the nongenetic components of life span are more influential than genetic factors in H. sapiens.
Results for investigations, dating back to 1903, that compare age of death in a parent with age of death in offspring have consistently shown very weak correlations. That is, contrary to popular belief, our life span is not determined by our parents. TABLE 7.1 shows the contribution of heredity to life span in a French Canadian population born during the nineteenth century. Although the contribution varies with age of death of the offspring and with gender, heredity accounts for no more than about 10–16% of the total influence on life span. Similar results have been found when evaluating the age of death in monozygotic (identical) and dizygotic (fraternal) twins: the contribution of heredity to life span was found to be no more that 25% in 2800 Danish twinpairs. Together, comparisons of the age of death of parents and their offspring and of twins strongly suggest that nongenetic factors influenced by intelligence may have had a significant effect on life span in H. sapiens. You‘ll learn the nature of these nongenetic factors in the next section.