第4讲 抗原提呈的魔力
HEADS UP!
For T cells to be activated, their receptors must recognize protein fragments presented by MHC molecules on the surface of special "antigen presenting cells." Presentation of antigen by class I MHC molecules lets killer T cells "look into" cells to determine if they are infected and should be destroyed. Presentation of antigen by class II MHC molecules alerts the immune system to invaders that don't infect cells, and helps guarantee that the decision to deploy the powerful adaptive immune system is not made by a single cell. Within the human population, there are genes for many slightly different MHC molecules. Consequently, it is likely that at least some humans will have MHC molecules which can display protein fragments from any pathogen.
INTRODUCTION
Of all the concepts on which the immune system is based, perhaps the most elegant, and certainly the most unexpected, is antigen presentation: the concept of having one cell present protein fragments to another cell. As you will see, antigen presentation is central to the function of the adaptive immune system, with the cells that present antigen to T cells – the antigen presenting cells (APCs) – playing a pivotal role. Let's begin by discussing the "billboards" on APCs that actually do the presenting: the class I and class II MHC molecules.
CLASS I MHC MOLECULES
The structures of class I and class II MHC molecules have now been carefully analyzed, so we have a good idea what these molecules look like. Class I molecules have a binding groove that is closed at both ends, so the small protein fragments (peptides) they present must fi t within the confines of the groove (the "bun," if you will). Indeed, when immunologists pried peptides from the grasp of class I molecules and sequenced them, they found that most of them are eight or nine amino acids in length. These peptides are anchored at the ends, and the slight variation in length is accommodated by letting the peptide bulge out a bit in the center.
Every human has three genes ( HLA-A, HLA-B, and HLA-C ) for class I MHC proteins situated on chromosome 6. Because we have two chromosome 6's (one from Mom and one from Dad), we all have a total of six class I MHC genes. Each class I HLA protein pairs with another
protein called β2-microglobulin to make up the complete class I MHC molecule. In the human population, there are a total of about 1,500 slightly different forms of the genes that encode the three class I HLA proteins. The proteins encoded by these variants of the HLA-A, HLA-B, and HLA-C genes have roughly the same shape, but they differ by one or a few amino acids. Immunologists call molecules that have many forms "polymorphic," and the class I HLA proteins certainly fi t this description. In contrast, all of us have the same gene for the β2-microglobulin protein.
Because they are polymorphic, class I MHC molecules can have different binding motifs, and therefore can present peptides which have different kinds of amino acids at their ends. For example, some class I MHC molecules bind to peptides that have hydrophobic amino acids at one end, whereas other MHC molecules prefer basic amino acids at this anchor position. Since humans have the possibility of expressing up to six different class I molecules, collectively our class I molecules can present a wide variety of peptides. Moreover, although MHC I molecules are picky about binding to certain amino acids at the ends of the peptide, they are rather promiscuous in their selection of amino acids at the center of the protein fragment. As a result, a given class I MHC molecule can bind to and present a large number of different peptides, each of which "fits" with the particular amino acids present at the ends of its binding groove.
CLASS II MHC MOLECULES
Like class I molecules, the class II MHC molecules (encoded by genes in the HLA-D region of chromosome 6) are wildly polymorphic. Within the human population, there are about 700 different versions of the class II MHC molecules. In contrast to class I MHC molecules, the binding groove of class II MHC molecules is open at both ends, so a peptide can hang out of the groove. As you might expect from this feature, peptides that bind to class II molecules are longer than those that occupy the closed groove of class I molecules – in the range of 13–25 amino acids. Further, for class II MHC molecules, the critical amino acids that anchor the peptides are spaced along the binding groove instead of being clustered at the ends.
ANTIGEN PRESENTATION BY CLASS I MHC MOLECULES
MHC I molecules are billboards that display on the surface of a cell, fragments of proteins manufactured by that cell. Immunologists call these endogenous proteins. They include ordinary cellular proteins such as enzymes and structural proteins, as well as proteins encoded by viruses and other parasites that may have infected the cell. For example, when a virus enters a cell, it uses the cellular biosynthetic machinery to produce proteins encoded by viral genes. A sample of these viral proteins is then displayed by class I MHC molecules along with samples of all the normal cellular proteins. So in effect, the MHC I billboards advertise a sample of all the proteins that are being made inside a cell. Almost every cell in the human body expresses class I molecules on its surface, although the number of molecules varies from cell to cell. Killer T cells (also called cytotoxic lymphocytes or CTLs) inspect the protein fragments displayed by class I MHC molecules. Consequently, almost every cell is an "open book" that can be checked by CTLs to determine whether it has been invaded by a virus or other parasite and should be destroyed. A typical human cell has about 100,000 class I molecules on its surface, and after they have been there for about a day, the MHC billboards are replaced by new ones – so the class I MHC display is kept current.
The way endogenous proteins are processed and loaded onto class I MHC molecules is very interesting. When mRNA is translated into protein in the cytoplasm of a cell, mistakes are frequently made. These mistakes can result in the production of useless proteins that don't fold up correctly. In addition, proteins suffer damage due to normal wear and tear. So to make sure our cells don't fill up with defective proteins, old or useless proteins are rapidly fed into protein-destroying "machines" in the cytoplasm that function rather like wood chippers.
These protein chippers are called proteasomes, and they cut proteins up into peptides. Most of these peptides are then broken down further into individual amino acids, which are reused to make new proteins. However, some of the peptides created by the proteasomes are carried by specific transporter proteins (TAP1 and TAP2) across the membrane of the endoplasmic reticulum (ER) – a large, sack-like structure inside the cell from which most proteins destined for transport to the cell surface begin their journey.
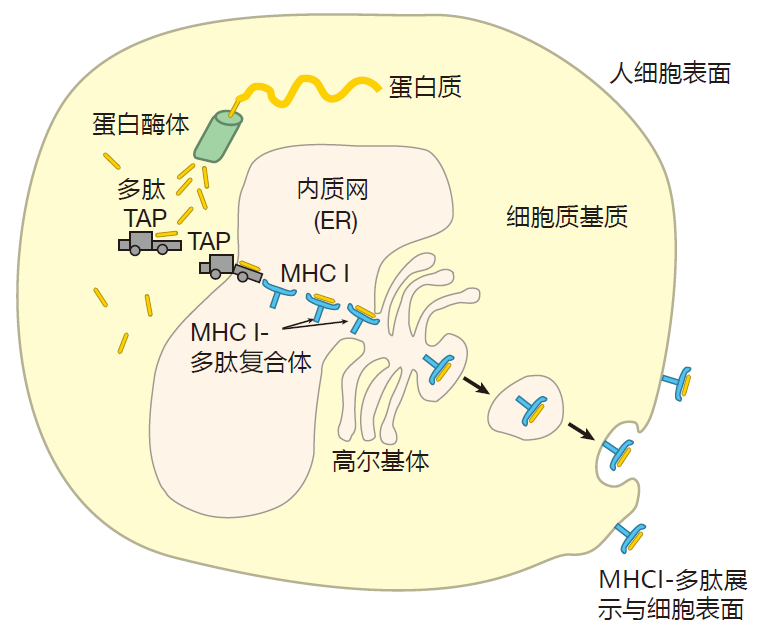
Once inside the ER, some peptides are chosen to be loaded into the grooves of class I MHC molecules. I say "chosen," because, as we discussed, not all peptides will fit. For starters, a peptide must be the right length – about nine amino acids. In addition, the amino acids at the ends of the peptide must be compatible with the anchor amino acids that line the ends of the groove of the MHC molecule. Obviously, not all of the "chips" prepared by the proteasome will have these characteristics, and those that don't are degraded or shipped back out of the ER into the cytoplasm. Once class I MHC molecules are loaded with peptides, they proceed to the surface of the cell for display. So there are three main steps in preparing a class I display: generation of a peptide by the proteasome, transport of the peptide into the ER by the TAP transporters, and binding of the peptide to the groove of the MHC I molecule.
In "ordinary" cells such as liver cells and heart cells, the major function of proteasomes is to deal with defective proteins. So as you can imagine, the chippers in these cells are not too particular about how proteins are cut up – they just hack away. As a result, some of the peptides will be appropriate for MHC presentation, but most will not be. In contrast, in cells such as macrophages that specialize in presenting antigen, this chipping is not so random. For example, binding of IFN-γ to receptors on the surface of a macrophage upregulates expression of three proteins called LMP2, LMP7, and MECL1. These proteins replace three "stock" proteins which are part of the normal proteasome machinery. The result of this replacement is that the "customized" proteasomes now preferentially cut proteins after hydrophobic or basic amino acids. Why, you ask? Because the TAP transporter and MHC I molecules both favor peptides that have either hydrophobic or basic C-termini. So in antigen presenting cells, standard proteasomes are modified so they will produce custom-made peptides, thereby increasing the efficiency of class I display.
Proteasomes also are not too particular about the size of peptides they make, and since the magic number for class I presentation is about nine amino acids, you might imagine that the ER would be flooded with useless peptides that are either too long or too short. However, it turns out that the TAP transporter has the highest affinity for peptides that are 8–16 amino acids long. Consequently, the TAP transporter screens peptides produced by proteasomes, and preferentially transports those that have the right kinds of C-termini and are approximately the correct length. Once candidate peptides have been transported into the ER, enzymes trim off excess N-terminal amino acids to make the peptide the right size for binding to class I MHC molecules.
An important feature of this "chop it up and present it" system is that the majority of proteins chopped up by proteasomes are newly made proteins which are structurally flawed – not old, worn-out proteins. Consequently, most peptides displayed by class I MHC molecules are derived from newly synthesized proteins, making it possible for the immune system to react quickly to an infection.
ANTIGEN PRESENTATION BY CLASS II MHC MOLECULES
Whereas class I MHC molecules are designed to present protein fragments to killer T cells, class II MHC molecules present peptides to helper T cells. And in contrast to class I MHC molecules, which are expressed on almost every kind of cell, class II molecules are expressed exclusively on cells of the immune system.
This makes sense. Class I molecules specialize in displaying proteins that are manufactured inside the cell, so the ubiquity of class I molecules gives CTLs a chance to check most cells in the body for infection. On the other hand, class II MHC molecules function as billboards that advertise what is happening outside the cell to alert helper T cells to danger. Therefore, relatively few cells expressing class II are required for this task – just enough to sample the environment in various parts of the body.
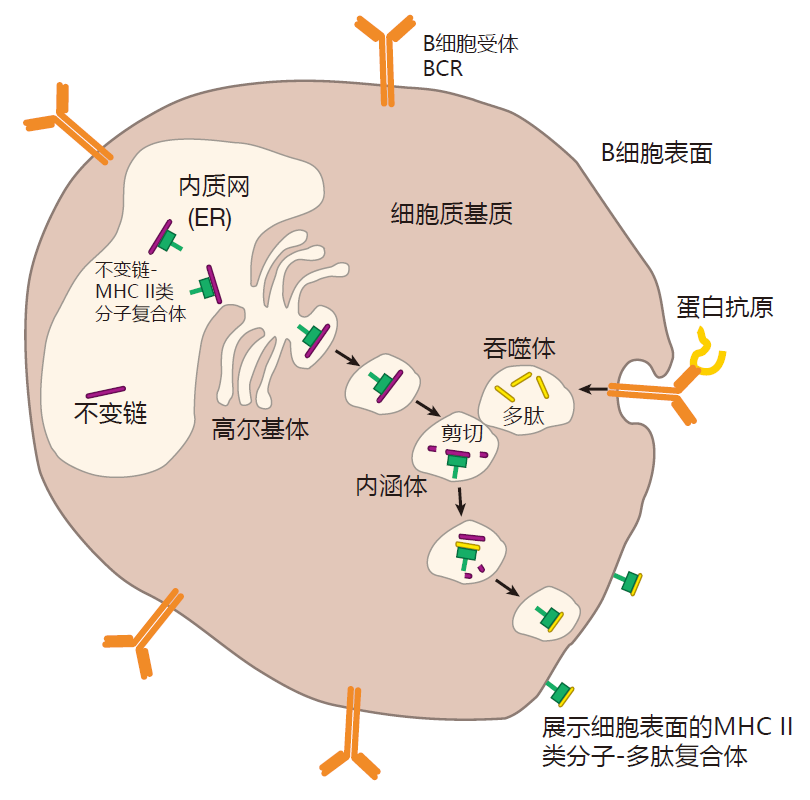
The two proteins that make up the class II MHC molecules (called the α and β chains) are produced in the cytoplasm and are injected into the endoplasmic reticulum where they bind to a third protein called the invariant chain. This invariant chain protein performs several functions. First, it sits in the groove of the MHC II molecule and keeps it from picking up other peptides in the ER.
This is important, because the ER is full of endogenous peptides that have been processed by proteasomes for loading onto class I MHC molecules. If these protein fragments were loaded onto class II molecules, then class I and class II MHC molecules would display the same kind of peptides: those made from proteins produced in the cell. Since the goal is for class II MHC molecules to present antigens that come from outside the cell, the exogenous proteins, the invariant chain performs an important function by acting as a "chaperone" that makes sure "inappropriate suitors" (endogenous peptides) don't get picked up by MHC II molecules in the ER.
Another function of the invariant chain is to guide class II MHC molecules out through the Golgi stack to special vesicles in the cytoplasm called endosomes. It is within endosomes that class II MHC molecules are loaded with peptides. The current thinking is that while class II MHC molecules are making their way from the ER to an endosome, proteins that are hanging around outside the cell are enclosed in a phagosome, and brought into the cell. This phagosome then merges with the endosome, and enzymes present in the endosome chop up the exogenous proteins into peptides. During this time, endosomal enzymes also destroy all of the invariant chain except for the piece called CLIP that actually is guarding the groove of the MHC molecule. Amazingly, although the exogenous proteins and the invariant chain are hacked to pieces by enzymes in the endosome, the class II MHC molecule itself remains unscathed. This is presumably because the MHC molecule is cleverly folded so that the enzymes can-not gain access to their favorite cleavage sites.
Meanwhile, a cellular protein called HLA-DM, which has also traveled to the endosome, catalyzes the release of CLIP, allowing an exogenous peptide to be loaded into the now-empty groove of the class II MHC molecule. But HLA-DM does more than just kick CLIP out to make room for the peptide. HLA-DM competes with potential peptides for binding to the class II MHC molecule, insuring that only peptides that bind tightly will be presented. Finally, the complex of MHC plus peptide is transported to the cell surface for display.
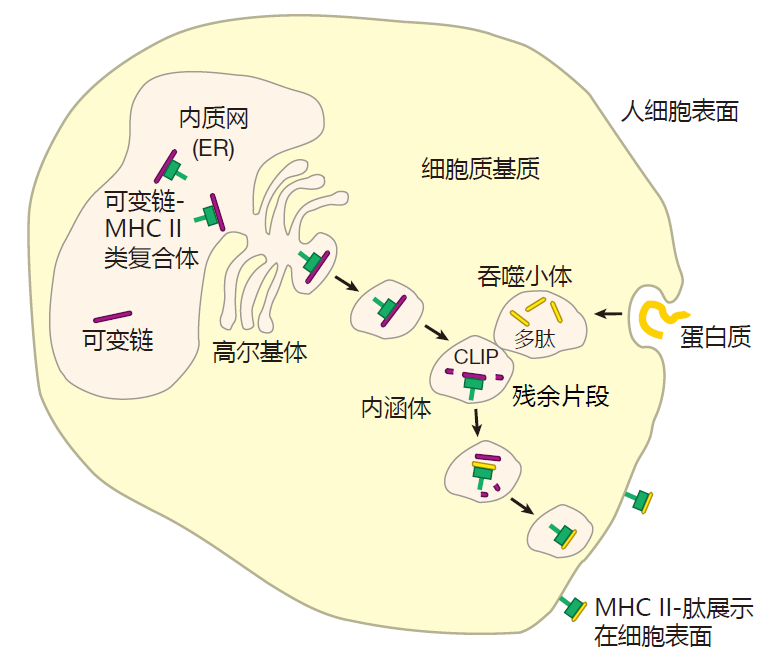
It is important to recognize that there are two separate loading sites and pathways for class I and class II MHC molecules. It is this separation of loading sites and pathways that allows the class I billboard to advertise what's going on inside the cell (for killer T cells) and the class II billboard to advertise what's happening outside (for helper T cells).
ANTIGEN PRESENTING CELLS
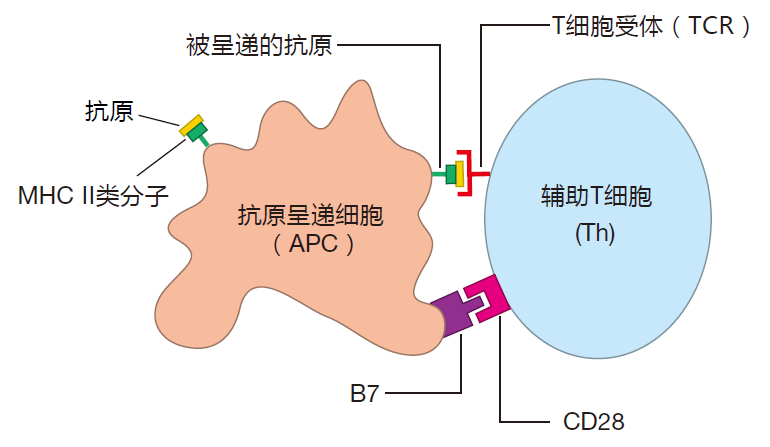
Before a killer T cell can kill or a helper T cell can help, it must be activated. For this to happen, a T cell must recognize its cognate antigen presented by an MHC molecule on the surface of another cell. But this is not enough. It must also receive a second, co-stimulatory signal. Only certain cells are equipped to provide both class I and class II MHC display as well as co-stimulation. These are the antigen presenting cells (APCs).
Because the job of antigen presenting cells is to activate killer and helper T cells, these cells really should have been named "T cell-activating cells." This would have avoided confusion with the "ordinary" cells in the body, which cannot activate T cells, but which do use class I MHC molecules to present antigens made inside these cells to alert killer T cells. Does it seem to you that immunologists just like to make things confusing? I sometimes think so. Anyway, to keep this straight, just remember that the term "antigen presenting cell" always refers to those special cells which can provide both the high levels of MHC proteins and the co-stimulatory molecules required for T cell activation.
Co-stimulation usually involves a protein called B7 on the surface of an antigen presenting cell that "plugs into" a protein called CD28 on the surface of a T cell.
Three types of antigen presenting cells have been identified: activated dendritic cells, activated macrophages, and activated B cells. All of these are white blood cells, and since new blood cells are made continuously, APCs can be replenished as needed.
Activated dendritic cells
Dendritic cells (DCs) have a characteristic starfish-like shape, and get their name from the word "dendrite," which is commonly used to describe the projections on nerve cells. It is important to note that these cells are very different from the plasmacytoid dendritic cells (pDCs) mentioned earlier – cells whose primary function is to produce large amounts of interferon α and β in response to a viral attack. In fact, pDCs are not even shaped like a starfish. They are round like plasma cells.
Dendritic cells were once considered to be only a curiosity. However, it is now appreciated that these cells are the most important of all the antigen presenting cells – because dendritic cells can initiate the immune response by activating virgin T cells. Here's how this works. The first DCs described were starfish-shaped "Langer-hans" cells that are found in the tissues just below the skin. However, dendritic cells have since been discovered all over the body. What is now clear is that these dendritic cells are sentinel cells which take up positions beneath the barriers of epithelial cells that represent our first line of defense. In normal tissues (tissues that have not been infected), DCs resemble wine tasters. Although they can take up about four times their volume of extracellular fluid per hour, they mostly just take it in and spit it back out. In this "resting" state, DCs express some B7 and relatively low levels of MHC molecules on their surface. As a result, these resting dendritic cells are not good at presenting antigen to T cells, especially to virgin T cells. This is because naive T cells require extensive receptor crosslinking by MHC–peptide complexes as well as powerful co-stimulation in order to be activated.
If there is a microbial invasion, and the tissues in which a dendritic cell resides become a battleground, the dendritic cell will become "activated." DCs can be activated by signals which come from other immune system cells that are engaged in battle. For example, both neutrophils and macrophages give off tumor necrosis factor (TNF) when they are trying to destroy an attacker, and this battle cytokine can activate DCs. Also, interferon α or β given off by virus-infected cells can trigger DC activation. Finally, dendritic cells have pattern-recognition receptors (e.g., Toll-like receptors) which they use to recognize molecular patterns that are characteristic of broad classes of invaders. The signals received by these pattern-recognition receptors can play important roles in activating dendritic cells.
Dendritic cells travel
When a dendritic cell is activated by battle cytokines, chemicals given off by dying cells, ligation of its pattern-recognition receptors, or a combination of these signals, the lifestyle of this "wine taster" changes dramatically. No longer does the dendritic cell "sip and spit." Now it "swallows" what it has taken in. Typically, a DC remains in the tissues for about six hours after activation, collecting a representative sample of battle antigens. At that point, phagocytosis ceases, and the pathogen-activated dendritic cell leaves the tissues and travels through the lymphatic system to the nearest lymph node. It is its ability to "travel when activated" which makes the dendritic antigen presenting cell so special.
Inside a resting DC are large numbers of "reserve" class II MHC molecules. When a resting DC is activated and starts to "mature," these class II MHC molecules begin to be loaded with antigens from the battle scene. And by the time a DC reaches its destination – a trip that usually takes about a day – these battle antigen-loaded class II MHC molecules will be prominently displayed on the surface of the cell. Also during its journey, the DC upregulates expression of its class I MHC molecules. Consequently, if the dendritic cell was infected by a virus out at the battle scene, by the time it reaches a lymph node, fragments of viral proteins will be on display on the dendritic cell's class I MHC billboards. Finally, while traveling, the DC increases production of B7 co-stimulatory proteins. So when it reaches a lymph node, the mature dendritic cell has everything it needs to activate virgin T cells: high levels of class I and class II MHC molecules loaded with the appropriate peptides, and plenty of B7 proteins.
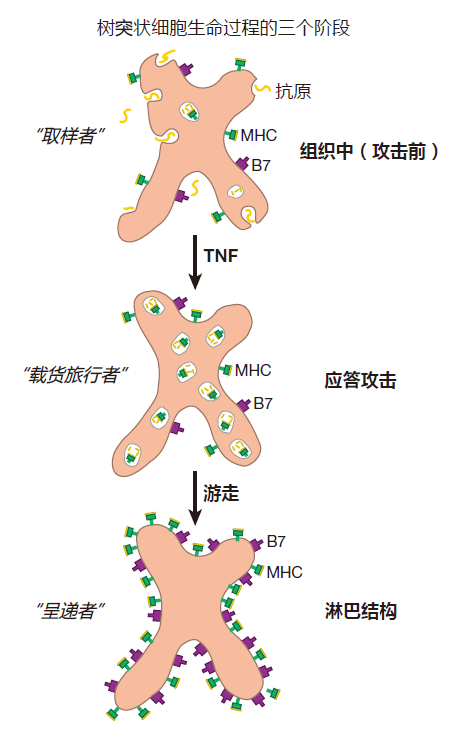
Now, why do you think DCs, which wildly sample antigens out in the tissues, stop their sampling when they begin their journey to a lymph node? Of course. Dendritic cells take a "snapshot" of what is happening on the "front lines," and carry this image to a lymph node – the place where virgin T cells congregate. There the traveling dendritic cells activate those virgin T cells whose T cell receptors recognize the invader that is "in the picture." The fact that battle cytokines such as TNF trigger the migration of DCs to a lymph node also makes perfect sense. After all, you want DCs to mature, travel to lymph nodes, and present antigen only if a battle is on.
Once a DC reaches a lymph node, it only lives for about a week. This short lifetime may seem strange at first. After all, this doesn't give a dendritic cell very long to meet up with the "right" virgin T cell which is circulating through the lymph nodes, looking for its cognate antigen. However, dendritic cells can interact with hundreds or even thousands of T cells every hour, and their short presentation life insures that dendritic cells carry snapshots of the battle which are up-to-date. In addition, after a dendritic cell has been activated, but before it begins its travels, it produces special cytokines (chemokines) which encourage white blood cells called monocytes to leave the blood, enter the tissues, and become dendritic cells. Consequently, activated dendritic cells recruit their own replacements. These newly recruited DCs can carry fresh images of the battle to lymph nodes as the battle continues.
There is another reason for the short lifetime of dendritic cells. In Lecture 2, I mentioned that it is very important that the magnitude of an immune response be in proportion to the seriousness of the attack. The short lifetime of DCs helps make this happen. Here's how. During a microbial attack, the number of T cells activated will depend on the number of mature dendritic cells that bring news of the battle to nearby lymph nodes. If the attack is weak, relatively few battle cytokines will be produced by warring macrophages, and only a small number of dendritic cells will be dispatched with their cargo. And because these DCs only live a short time once they reach the lymph node, only a limited number of T cells will be activated – just enough to deal with the small number of microbial invaders. On the other hand, if the infection is serious, many battle cytokines will be produced, many dendritic cells will be activated and travel to nearby lymph nodes, many more DCs will be recruited from the blood, and many T cells will be activated. Consequently, one result of the dendritic cell's short lifetime is that the number of DCs in the lymph nodes at a given moment will reflect the current situation at the battle site, and the magnitude of the immune response will be in proportion to the severity of the infection.
So dendritic antigen presenting cells are sentinel cells that "sample" antigens out in the tissues. If there is an invasion, DCs become activated and travel to nearby lymph nodes. There they initiate the adaptive immune response by presenting antigen collected at the battle scene to virgin T cells. Activated DCs are short-lived, and the rapid turnover of these cells insures that the "pictures" they bring to a lymph node are continuously updated. Moreover, the number of dendritic cells dispatched from the tissues and the number of replacement dendritic cells recruited will depend on the severity of the attack. Consequently, the immune system is able to mount a response that is proportional to the danger posed by the invasion. Can you imagine a more ingenious system? I don't think so!
Dendritic cells are classified as part of the innate immune system because their receptors are "hard-wired" and not "adaptable" like those of B and T cells. However, as I'm sure you now understand, DCs actually function as a "bridge" between the innate and the adaptive systems.
Activated macrophages
Macrophages also are sentinel cells which stand guard over areas of our body that are exposed to the outside world. They can function as garbage collectors, antigen presenting cells, or ferocious killers – depending on the signals they receive from the microenvironment in which they reside. In a resting state, macrophages are good at tidying up, but they are not much good at antigen presentation. This is because macrophages only express enough MHC and co-stimulatory molecules to function as antigen presenting cells after they have been activated by battle cytokines such as IFN-γ, or by having their pattern-recognition receptors (e.g., their Toll-like receptors) ligated by invading pathogens.
So macrophages resemble dendritic cells in that they efficiently present antigen only when there is something dangerous to present. However, it is important to recognize that dendritic antigen presenting cells don't kill and macrophages don't travel. Indeed, DCs can be pictured as "photojournalists" who don't carry weapons, who take snapshots of the fighting, and who then leave the battlefield to file their stories. In contrast, macrophages are heavily armed soldiers who must stand and fight. After all, macrophages are one of our main weapons in the early defense against invaders. Nevertheless, their lack of mobility raises an interesting question: What good is the activated macrophage's capacity to present antigen if it can't travel to lymph nodes where virgin T cells are located? Here's the answer.
Once they have been activated by dendritic cells, T cells exit the lymph nodes, circulate through the blood, and enter inflamed tissues to help with the battle. However, these "experienced" T cells must be continually re-stimulated. Otherwise, they think the battle has been won, and they go back to a resting state or die of neglect. That's where activated macrophages come in. Out in the tissues, macrophages act as "refueling stations" which keep experienced T cells "turned on" so they can continue to participate in the battle. So mature dendritic cells activate virgin T cells, and activated tissue macrophages mainly function to re-stimulate experienced T cells.
Activated B cells
The third APC is the activated B cell. A virgin B cell is not much good at antigen presentation, because it expresses only low levels of class II MHC molecules and little or no B7. However, once a B cell has been activated, the levels of class II MHC molecules and B7 proteins on its surface increase dramatically. As a result, an experienced B cell is able to act as an antigen presenting cell for Th cells. B cells do not function as APCs during the initial stages of an infection, because at that time they are still naive – they haven't been activated. However, later in the course of the infection or during subsequent infections, presentation of antigen by experienced B cells plays an important role. This is because B cells have one great advantage over the other APCs: B cells can concentrate antigen for presentation. After a B cell's receptors have bound to their cognate antigen, the whole complex of BCR plus antigen is removed from the cell surface and dragged into the cell. There the antigen is processed, loaded onto class II MHC molecules, and transported back to the cell surface for presentation.
B cell receptors have a high affinity for antigen, so they act like "magnets," collecting antigen for presentation to Th cells. Because a threshold number of T cell receptors must be crosslinked by presented antigen before a Th cell can be activated, it is estimated that activated B cells have a 100- to 10,000-fold advantage over other APCs in activating helper T cells at times when there is relatively little antigen around. Presentation of antigen by B cells is also very fast. Less than half an hour elapses between the time antigen is captured by a B cell's receptors and the time it is displayed on the cell surface by class II MHC molecules.
In summary, when an invader is first encountered, all the B cells which could recognize that particular invader are virgins, so the important APCs are activated dendritic cells. Then, while the battle is raging, activated macrophages on the front lines present antigen to warring T cells to keep them pumped up. Later in the infection, or if this same invader is encountered again, experienced B cells are extremely important APCs – because they can quickly activate helper T cells by concentrating small amounts of antigen for presentation.
THE LOGIC OF CLASS I MHC PRESENTATION
To really appreciate why antigen presentation is one of Mother Nature's greatest "inventions," we need to think a little about the logic behind this amazing activity. For starters, we need to ask the question: Why bother with MHC presentation at all? Why not just let a T cell's receptors recognize un-presented antigen the way a B cell's receptors do? This is really a two-part question, since we are talking about two rather different displays: class I and class II. So let's examine these one at a time.
One reason for class I presentation is to focus the attention of killer T cells on infected cells, not on viruses and other pathogens that are outside our cells in the blood and tissues. So long as pathogens remain outside of our cells, antibodies can tag them for destruction by professional phagocytes, and can bind to them and prevent them from initiating an infection. Since each plasma B cell can pump out about 2,000 antibody molecules per second, these antibodies are "cheap" weapons that deal quite effectively with extracellular invaders. However, once microbes enter a cell, antibodies can't get at them.
When this happens, killer T cells – the high-tech, "expensive" weapons, specifically designed to destroy infected cells – are needed. And the requirement that killer T cells recognize antigens presented by class I MHC molecules on infected cells insures that CTLs won't waste their time going after invaders that are outside of cells – invaders which antibodies usually can deal with quite effectively.
In addition, it would be extremely dangerous to have un-presented antigen signal T cell killing. Imagine how terrible it would be if uninfected cells happened to have debris from dead viruses stuck to their surface, and killer T cells recognized this un-presented antigen and killed those "innocent bystander" cells. That certainly wouldn't do.
Another reason class I display is so important is that most proteins made in a pathogen-infected cell remain inside the cell, and never make their way to the cell surface. So without class I display, many pathogen-infected cells would go undetected by killer T cells. In fact, part of the magic of the class I MHC display is that, in principle, every protein of an invading pathogen can be chopped up and displayed by class I MHC molecules for killer T cells to view.
Finally, because their receptors recognize "native" antigens that have not been fragmented and presented, B cells actually are at a disadvantage. The reason is that most proteins must be folded in order to function properly. As a result of this folding, many epitopes that a B cell's receptors might recognize are unavailable for viewing – because they are on the inside of a folded protein molecule. In contrast, when a protein is chopped up into short pieces and presented by class I MHC molecules, epitopes can-not be hidden from killer T cells.
So the logic of class I MHC presentation is easy to understand, but why are MHC molecules so polymorphic? After all, there are so many different forms in the human population that most of us inherit genes for six different class I molecules. Doesn't this seem a bit excessive?
Well, suppose there were only a few different class I MHC proteins. Now imagine what might happen if a virus were to mutate so that none of its peptides would bind to any of these MHC I molecules. Such a virus might wipe out the entire human population, because no killer T cells could be activated to destroy virus-infected cells. So polymorphic MHC molecules give at least some people in the population a chance of surviving an attack by a clever pathogen.
Okay, but why do we have six genes for class I MHC proteins? That seems like a lot, especially since the class I MHC proteins are so polymorphic. The answer is that the possibility of "owning" up to six different class I MHC molecules increases the probability that each of us, individually, will have at least one class I MHC molecule into which a given pathogen's protein fragments will fit. Indeed, AIDS patients who have inherited the maximum number of different class I MHC molecules (six) live significantly longer on average than do patients who have genes encoding only five or fewer different class I molecules. The thinking here is that as the AIDS virus mutates, having a larger number of different class I molecules increases the probability that mutated viral proteins can be presented. Why six, not ten, genes for class I MHC molecules? I haven't a clue!
THE LOGIC OF CLASS II MHC PRESENTATION
Okay, so class I MHC presentation makes a lot of sense. But what about class II presentation? At first glance, this "dual display" (class I and class II) by antigen presenting cells might seem overly complicated. What must be appreciated, however, is that many pathogens do not infect human cells: They are quite happy living and reproducing outside our cells in tissues or in blood. If antigen presenting cells could only display proteins made by pathogens that infect them, intelligence on many of the most dangerous microbes would never reach the command centers in lymph nodes. By using class II MHC molecules to advertise a sampling of the total environment at the battle front, intelligence on all types of invaders can be made available to helper T cells.
But couldn't helper T cells just recognize unpresented antigen? After all, they aren't killers, so there isn't the problem of bystander killing. That's true, of course, but there is still a safety issue here. Antigen presenting cells only present antigen efficiently when a battle is going on, and helper T cells are screened to be sure that they do not react against our own proteins.
Consequently, both the helper T cell and the antigen presenting cell must "agree" that there has been an invasion before a helper T cell can be activated. So the requirement that helper T cells only recognize presented antigen insures that the decision to deploy the potentially deadly adaptive immune system is not made by a single cell.
Also, like class I molecules, class II molecules present small fragments of proteins. As a result, the number of targets that a helper T cell can "see" during presentation far exceeds those available for viewing in a large, folded protein. The consequence of this expanded number of targets is a stronger, more diverse immune reaction in which many different helper T cells will be activated – helper T cells whose receptors recognize the many different epitopes that make up the antigens of an invader.
CROSS-PRESENTATION
Although the separation of class I and class II pathways is the "law," it has been shown that a certain subset of antigen presenting cells can take up exogenous antigens and shuttle them into the class I pathway for presentation by class I MHC molecules. Such an unlawful use of the class I display has been termed cross-presentation. The idea is that if a clever pathogen (e.g., a virus) figured out a way to avoid infecting antigen presenting cells, yet could still infect and reproduce in other cells of the body, cross-presentation would give the immune system a chance to activate CTLs against this pathogen. So far, the rules that govern cross-presentation have not been clearly defined, and it is not known whether cross-presentation by class I MHC molecules of antigen taken up from outside an APC is an important feature of the human immune system.
NON-CLASSICAL MHC MOLECULES AND LIPID PRESENTATION
Class I and class II MHC molecules are called classical MHC molecules. So as you might expect, there also are non-classical MHC molecules. The best studied of these is the CD1 family of proteins. These non-classical MHC molecules resemble class I MHC molecules in that they consist of a long, heavy chain protein which is paired with the β2-microglobulin protein. However, in contrast to classical MHC molecules, which have grooves that are suitable to bind short peptides, the CD1, non-classical MHC molecules have grooves which are designed to bind lipids. CD1 molecules can "sample" lipids from various compartments within a cell, and can present these molecules on the surface of antigen presenting cells, where they can activate T cells. Consequently, it has been postulated that these non-classical MHC molecules give T cells a way of surveying the lipid composition of cells, just as class I MHC molecules allow T cells to examine a cell's proteins.
For every rule in immunology there seems to be an exception, and the rule has been that T cells only recognize fragments of proteins presented by class I and class II MHC molecules. Obviously, CD1 presentation of lipids to T cells is an exception to this rule. So far, however, it is not clear how important lipid presentation is for the immune defense. Consequently, I will "stick to the rule" that T cells only recognize protein antigens. Be aware, however, that this may change as more research is done on CD1-presented lipids.
MHC PROTEINS AND ORGAN TRANSPLANTS
In addition to their "natural" role in antigen presentation, MHC molecules also are important in the "unnatural" setting of organ and tissue transplantation. Transplantation studies actually began in the 1930s with experiments involving mouse tumors. In those days, tumors were usually induced by rubbing some horrible chemical on the skin of a mouse, and then waiting for a long time for a tumor to develop. Because it was so much trouble to make these tumors, biologists wanted to keep the tumor cells alive for study after the mouse had died. They did this by injecting some of the tumor cells into another, healthy mouse, where the cells would continue to grow. What they observed, however, was that the tumor cells could only be successfully transplanted when the donor and recipient were from a strain of mice in which there had been a lot of inbreeding. And the more inbred the strain, the better the chance for survival of the transplant. This provided the impetus for the creation of the many inbred mouse strains that immunologists depend on today.
Once inbred mouse strains were available, immunologists began to study the transplantation of normal tissues from one mouse to another. Right away they noticed that if a small patch of skin from one mouse was grafted onto the skin of another mouse, this new skin retained its healthy pink color and continued to grow – so long as the two mice were from the same inbred strain. In contrast, when this experiment was tried with mice that were not inbred, the transplanted skin turned white within hours (because the blood supply had been cut off) and invariably died. Immunologists figured this immediate graft rejection must be due to some genetic incompatibility, because it did not occur with inbred mice that have the same genes. To identify the genes involved in "tissue compatibility" (histocompatibility), immunologists bred mice to create strains that differed by only a few genes, yet which were still incompatible for tissue transplants. Whenever they did these experiments, they kept identifying genes that were grouped in a complex on mouse chromosome 17 – a complex they eventually called the "major histocompatibility complex" or MHC.
So the MHC molecules that we have been discussing in the context of antigen presentation are the very same molecules that are responsible for immediate rejection of transplanted organs. It turns out that killer T cells are particularly sensitive to MHC molecules that are "foreign," and when they see them, they attack and kill the cells that express them. Some of their favorite targets are the cells that make up the blood vessels contained within the donated organ. By destroying these vessels, CTLs cut off the blood supply to the transplanted organ, usually resulting in its death. For this reason, transplant surgeons try to match donors and recipients who have the same MHC molecules. However, finding such a match is difficult. Indeed, it is estimated that if you had access to organs contributed by 10,000,000 different individuals who were not related to you, the chance of your finding an exact match to all your class I and class II MHC molecules would only be about 50%. So the diversity of MHC molecules, which is so important in protecting us from new or mutated invaders, creates a real problem for organ transplantation. Clearly, the immune system did not evolve with organ swapping in mind!
REVIEW
Class I MHC molecules function as billboards that display what is going on inside a cell. For example, when a virus infects a cell, it uses that cell's biosynthetic machinery to produce viral proteins. Some of these proteins are cut up into small pieces (peptides) by the proteasome, and carried by the TAP transporters into the endoplasmic reticulum (ER). There the peptides are "interviewed" by class I molecules. Those that are about nine amino acids in length with appropriate amino acids at their ends are bound in the grooves of class I MHC molecules, and are transported to the surface of the cell. By scanning the MHC I–peptide complexes displayed there, killer T cells can "look into a cell" to determine whether it has been infected and should be destroyed.
Class II MHC molecules also are billboards, but they are designed to alert helper T cells that a battle is being waged. Class II molecules are assembled in the ER, just like class I molecules, but because invariant chain proteins occupy their binding grooves, class II molecules do not pick up peptides in the ER. Instead, the class II–invariant chain complex is transported out of the ER and into another cellular compartment called an endosome. There they meet up with proteins that have been taken into the cell by phagocytosis and cut up into peptides by enzymes.
These peptides then replace the invariant chains that have been guarding the grooves of the class II molecules, and the MHC–peptide complexes are transported to the cell surface for display to Th cells. By this clever mechanism, class II molecules pick up peptides derived from proteins taken in from outside the cell, but avoid peptides derived from proteins made within the cell.
The display by MHC molecules of fragmented proteins has several advantages over a display of intact proteins. First, most viral proteins normally remain hidden inside an infected cell and are not found on the cell surface. Therefore, these proteins would never be seen by killer T cells unless they were advertised by class I MHC molecules. In addition, because protein folding can hide large portions of a protein from view, chopping a protein up into small peptides reveals many potential T cell targets that would be inaccessible in an intact protein. Consequently, MHC display greatly increases the probability that CTLs will recognize an infected cell, and that helper T cells will be alerted to a microbial attack.
Both class I and class II MHC molecules are extremely polymorphic, and humans have multiple genes for both classes of MHC molecules. Consequently, it is likely that your MHC molecules will be able to display peptides from most pathogens, and that at least some people in the population will have MHC molecules capable of displaying peptides from any pathogen.
Antigen presenting cells are special immune system cells that can provide both class I and class II MHC display as well as co-stimulation. The most important antigen presenting cell during the initial stages of an attack is the dendritic cell, because this cell can activate virgin T cells. When a DC detects danger signals at the scene of the battle, it begins to mature and migrates with its cargo of "battle antigen" to a nearby lymph node. There, the dendritic cell uses class II MHC molecules to display fragments of the proteins it has collected out in the tissues, and class I MHC molecules to display fragments of proteins made by viruses or bacteria that may have infected the dendritic cell out at the battle site. In this way, the dendritic cell effectively takes a snapshot of what is going on at the front, carries it to the place where T cells are plentiful, and then does its "show and tell" thing to activate T cells.
Macrophages, activated by danger signals, also can function as antigen presenting cells. However, activated macrophages don't travel to lymph nodes to present antigen. They stay put in the tissues and battle invaders. Consequently, macrophages are most useful for presenting antigen after the adaptive immune system has been activated. At that time, activated macrophages out in the tissues can keep experienced T cells fi red up, prolonging the time that they are effective in dealing with invaders.
Activated B cells are the third type of antigen presenting cell, but again, these cells aren't useful in initiating the adaptive response against a new invader. The reason is that before B cells can function as antigen presenting cells, they must first be activated by helper T cells – and Th cells must wait to be activated by dendritic cells. So B cells don't get "certified" to be antigen presenting cells until after the adaptive immune response has already fi red up. Nevertheless, once activated, B cells have a great advantage over DCs and macrophages: B cells can use their receptors as "antigen collectors" to concentrate small amounts of antigen for presentation to helper T cells. Consequently, relatively late in the initial infection or early in a subsequent infection by the same attacker, B cells play a major role as antigen presenting cells.
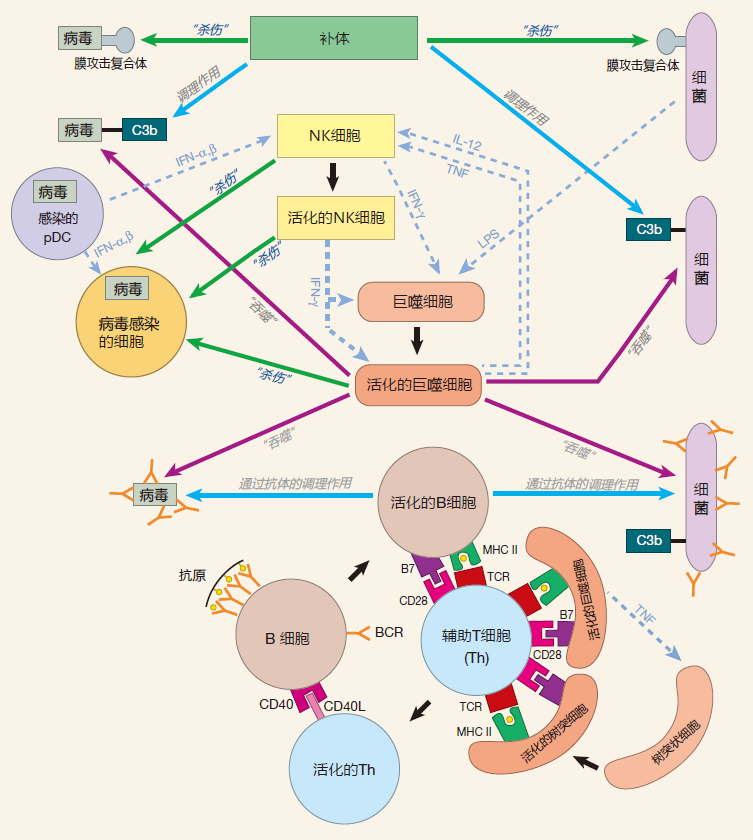
SUMMARY FIGURE
You will notice that our summary figure now includes antigen presenting cells with their MHC and B7 molecules.