第5讲 T Cell Activation
HEADS UP!
Before they can do any work, T cells must be activated. This requirement helps insure that T cells will spring into action only when there is real danger, and that only useful weapons will be mobilized. T cell activation requires recognition of the invader by the T cell's receptors, the function of co-receptor molecules that focus the attention of TCRs on the appropriate MHC molecule (class I or class II), and co-stimulation provided by an activated antigen presenting cell. There are many similarities between the ways B cells and T cells are activated – but there are also important differences.
INTRODUCTION
The innate immune system maintains large stockpiles of weapons. This makes sense because common invaders are attacking our bodies almost continuously, and the weapons of the innate immune system are useful against a wide variety of these "everyday" enemies. In contrast, only about one in a million B or T cells will have receptors that can recognize a given invader. Consequently, it would not be wise to stockpile B or T cells, because in our entire lifetime, we probably will never encounter the invader which a particular B or T cell could defend against. Indeed, an important feature of the adaptive immune system is that its weapons are made on demand: Only those B and T cells whose receptors can recognize the "invader du jour " are mobilized. The first step in mobilizing these weapons is activation, and in this lecture, we're going to focus on how T cells are activated. What they do once they are activated will be the subject of the next lecture.
T CELL RECEPTORS
T cell receptors (TCRs) are molecules on the surface of a T cell that function as the cell's "eyes" on the world. Without these receptors, T cells would be flying blind with no way to sense what's going on outside. T cell receptors come in two flavors: αβ and γδ. Each type of receptor is composed of two proteins, either α and β or γ and δ. Like the heavy and light chains of the B cell receptor, the genes for α, β, γ, and δ are assembled by mixing and matching gene segments. In fact, in B and T cells, the same proteins ( RAG1 and RAG2 ) initiate the splicing of gene segments by making double-stranded breaks in chromosomal DNA. As the gene segments are mixed and matched, a "competition" ensues from which each T cell emerges with either an αβ or a γδ receptor, but not both. Generally, all the TCRs on a mature T cell are identical – although there are exceptions to this rule.
Traditional T cells
Over 95% of the T cells in circulation have αβ T cell receptors, and express either CD4 or CD8 "co-receptor" molecules (more about these co-receptors in a bit). The αβ receptors of these "traditional" T cells recognize a complex composed of a peptide and an MHC molecule on the surface of a cell. Each "mature" T cell will have receptors that recognize peptides associated either with class I MHC molecules or with class II MHC molecules. Importantly, the αβ receptors of a traditional T cell recognize both the peptide and the MHC molecule and, unlike B cells, T cells cannot undergo hypermutation to change the affinity of their TCRs for their cognate antigen.
Non-traditional T cells
In addition to traditional T cells, several kinds of "nontraditional" T cells have been discovered. T cells which have γδ receptors are considered to be non-traditional because, in contrast to traditional T cells, most γδ T cells do not express either the CD4 or CD8 co-receptor molecules. T cells with γδ receptors are most abundant in areas such as the intestine, the uterus, and the tongue, which are in contact with the outside world. Interestingly, mice have lots of γδ T cells in the epidermal layer of their skin, but humans do not. This serves to remind us that so far as the immune system is concerned, humans are not just big mice. After all, human and mouse lineages diverged roughly 65 million years ago, and humans are relatively large animals that can live for a long time. In contrast, mice are small and short-lived. An "elderly" mouse is about two years old! Consequently, we would predict that the immune systems which evolved to protect humans and mice, although similar, would be different. And they are.
Although αβ TCRs are thought to be about as diverse as BCRs, γδ receptors are much less diverse. Moreover, the receptors of γδ T cells in the tongue and uterus tend to favor certain gene segments during rearrangement, whereas γδ receptors in the intestine prefer other combinations of gene segments. The thinking here is that, like players on the innate immune system team, γδ T cells stand watch on the "front lines," and have receptors which are "tuned" to recognize invaders that usually enter at certain locations.
There is a lot about γδ T cells which is still mysterious. For example, it is not known where these cells grow up. Traditional T cells mature in the thymus, and although γδ T cells also are found in the thymus, nude mice, which lack a thymus, still produce functional γδ T cells. In most cases, it also is not known exactly what the receptors on γδ T cells recognize, but it is believed that, like B cells, γδ T cells focus on un-presented antigen. The receptors of some γδ T cells recognize proteins (e.g., MICA and MICB) which are expressed on the surface of cells that are under stress. Consequently, it has been postulated that γδ T cells are designed to kill cells that become stressed as the result of a microbial infection. Nevertheless, the exact mission of γδ T cells is not clear.
There is another type of non-traditional T cell that is mentioned frequently, but about which relatively little is known: the NKT cell. In a human, only about 1% of the T cells in the blood are of this type. As its name implies, this non-traditional T cell has some of the properties of the natural killer (NK) cells of the innate system, and some of the properties of traditional T cells of the adaptive immune system. NKT cells mature in the thymus and have αβ receptors. However, in contrast to the αβ receptors of traditional T cells, which are incredibly diverse, the repertoire of receptors expressed by NKT cells is quite limited. In addition, the receptors of NKT cells recognize lipids presented by non-classical CD1 MHC molecules instead of protein fragments presented by class I or class II MHC molecules. It has been proposed that NKT cells evolved as a weapon designed to protect us against microbes such as tuberculosis which produce characteristic lipid molecules. However, normal mice and mice that have been engineered to lack NKT cells are equally susceptible to infection with TB, and, so far, it is not clear how important NKT cells are in protecting humans against bacterial infections.
Because much more is known about traditional T cells than about their non-traditional cousins, and because traditional T cells seem to be the ones that are most important for protecting us from disease, we will limit our discussion in this book to T cells of the traditional variety.
HOW A T CELL'S RECEPTORS SIGNAL
Once a TCR has recognized its cognate antigen presented by an MHC molecule, the next step is to transmit a signal from the surface of the T cell, where recognition takes place, to the nucleus of the T cell. For the T cell to switch from a resting state to a state of activation, gene expression must be altered, and these genes are, of course, located in the cell's nucleus. Normally, this type of signaling across the cell membrane involves a transmembrane protein that has two parts: an external region which binds to a molecule (called a ligand) that is outside the cell, plus an internal region that initiates a biochemical cascade which conveys the "ligand bound" signal to the nucleus. Here the TCR runs into a bit of a problem. As is true of the BCR, the αβ TCR has a perfectly fine extracellular domain that can bind to its ligand (the combination of MHC molecule and peptide), but the cytoplasmic tails of the α and β proteins are only about three amino acids long – much too short to signal.
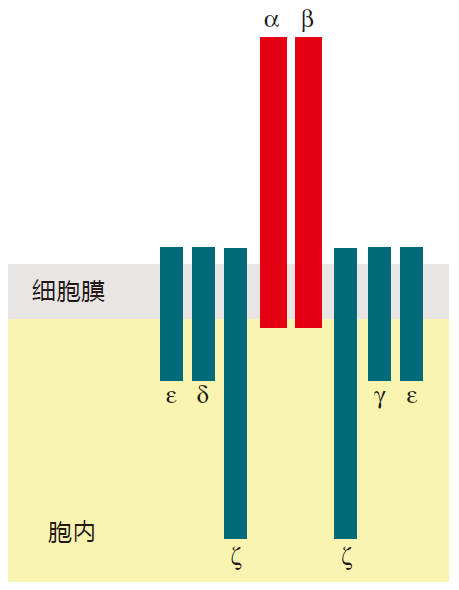
To handle the signaling chores, a few bells and whistles had to be added to the TCR: a complex of proteins collectively called CD3. In humans, this signaling complex is made up of four different proteins: γ, δ, e, and ζ (gamma, delta, epsilon, and zeta). The CD3 proteins are anchored in the cell membrane, and have cytoplasmic tails that are long enough to signal just fine. Please note, however, that the γ and δ proteins that are part of the CD3 complex are not the same as the γ and δ proteins that make up the γδ T cell receptor.
The whole complex of proteins (α, β, γ, δ, e, ζ) is transported to the cell surface as a unit. If any one of these proteins fails to be made, you don't get a TCR on the surface. Consequently, most immunologists consider the functional, mature TCR to be this whole complex of proteins. After all, the α and β proteins are great for recognition, but they can't signal. And together, the γ, δ, e, and ζ proteins signal just fine, but they are totally blind to what's going on outside the cell. You need both parts to make it work. As with BCRs, signaling involves clustering TCRs together in one area of the T cell surface. When this happens, a threshold number of kinase enzymes are recruited by the cytoplasmic tails of the CD3 proteins, and the activation signal is dispatched to the nucleus.
When the α and β chains of the TCR were first discovered, it was thought that the TCR was just an on/off switch whose only function was to signal activation. But now that you have heard about the CD3 proteins, let me ask you: Does this look like a simple on/off switch? No way! This TCR can send signals that result in very different outcomes, depending on how, when, and where it is triggered. For example, in the thymus, if a T cell's receptors recognize MHC plus self peptide, the TCRs can trigger the T cell to commit suicide to prevent autoimmunity. Later in its life, if its TCRs recognize their cognate antigen presented by MHC molecules, but that T cell does not receive the required co-stimulatory signals, the T cell may be neutered (anergized) so it can't function. And, of course, when a TCR is presented with its cognate antigen, and co-stimulatory signals are available, the TCR can signal activation. So this same T cell receptor, depending on the situation, signals death, anergy, or activation. In fact, there are now documented cases in which the alteration of a single amino acid in a presented peptide can change the signal from activation to death! Clearly this is no on/off switch, and immunologists are working very hard to understand exactly how TCR signaling is "wired," and what factors influence the signaling outcomes.
CD4 AND CD8 CO-RECEPTORS
In addition to the T cell receptor, there are two more molecules which are involved in antigen recognition by T cells – the CD4 and CD8 co-receptors. Now, doesn't it seem that Mother Nature got carried away when she added on these CD4 and CD8 co-receptors? I mean, there are already two proteins, α and β, to use for antigen recognition, and four more, γ, δ, e, and ζ, to use for signaling. Wouldn't you think that would do it? Apparently not, so there must be essential features of the system that require CD4 and CD8 co-receptors. Let's see what these might be.
Killer T cells and helper T cells perform two very different functions, and they "look at" two different molecules, class I or class II MHC, respectively, to get their cues. But how do CTLs know to focus on peptides presented by class I molecules – and how do Th cells know to scan APCs for peptides presented by class II? After all, it wouldn't be so great if a CTL got confused, recognized a class II–peptide complex on an APC, and killed that antigen presenting cell. So here's where CD4 and CD8 come in.
When T cells begin maturing in the thymus, they express both types of co-receptors on their surface. Immunologists call them CD4 + CD8 + or "double-positive" cells. Importantly, CD4 co-receptors will only "clip onto" class II MHC molecules, and CD8 co-receptors will only match with class I MHC molecules. So CD4 and CD8 co-receptors help focus the attention of Th cells and CTLs on the proper MHC molecule. The latest thinking is that double-positive T cells scan APCs, looking for MHC molecules that their TCRs can bind to. If a T cell's receptors have the right shape to bind to class II MHC molecules on the surface of an APC, the CD4 co-receptors will clip on. Conversely, if the TCRs are shaped properly to bind to class I MHC molecules, the CD8 co-receptors will connect.
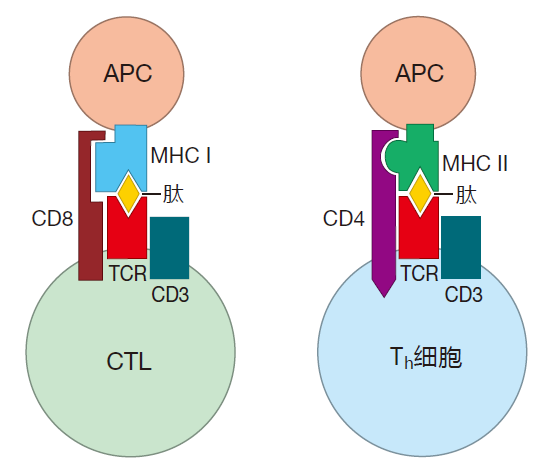
CD4 and CD8 molecules have tails that extend through the T cell's plasma membrane and into the cytoplasm. Although these tails are different, they both have the right characteristics to signal. So when a CD4 molecule clips onto a class II MHC molecule, expression of the CD8 co-receptor is downregulated, and the T cell becomes a "single-positive" CD4 + T cell that is committed to function as a helper T cell. In contrast, if the CD8 co-receptor clips onto the class I MHC molecule, CD4 expression is terminated, and that cell becomes a killer T cell. That's the idea, but exactly how these co-receptor molecules help "instruct" T cells to function as helpers or killers is not known.
CO-STIMULATION
In naive T cells, the "connection" between the T cell's receptors and the cell's nucleus is not very good. It's as if the T cell had an electrical system in which a large resistor were placed between the sensor (the TCR) and the piece of equipment it is designed to regulate (gene expression in the nucleus). Because of this "resistor," a lot of the signal from the TCR is lost as it travels to the nucleus. The result is that a prohibitively large number of TCRs would have to engage their cognate antigen before the signal that reaches the nucleus would be strong enough to have any effect. If, however, while the TCRs are engaged, the T cell also receives co-stimulation, the signal from the TCRs is amplified many times, so that fewer (probably about 100-fold fewer) TCRs must be engaged to activate a naive T cell. Although a number of different molecules have been identified which can co-stimulate T cells, certainly the best studied examples are the B7 proteins (B7–1 and B7–2) which are expressed on the surface of antigen presenting cells. B7 molecules provide co-stimulation to T cells by plugging into receptor molecules called CD28 on the T cell's surface.
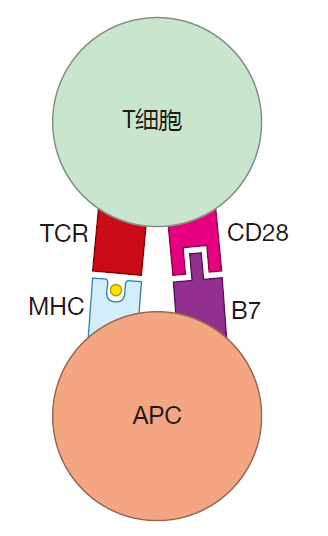
So, in addition to having their T cell receptors ligated by MHC–peptide, naive T cells also must receive co-stimulatory signals before they can be activated. Co-stimulation can be thought of as an "amplifier" that strengthens the "I'm engaged" signal sent by a T cell's receptors, thereby lowering the threshold number of TCRs which must be crosslinked by MHC–peptide complexes. Interestingly, once a naive T cell has been activated, the connection between the TCRs and the nucleus strengthens. It is as if an experienced T cell has been "re-wired" so that the resistor present in a naive T cell is bypassed. As a result of this re-wiring, amplification of the TCR signal is not as important for an experienced T cell as it is for a virgin T cell. Consequently, experienced T cells have a reduced requirement for co-stimulation.
A TIME-LAPSE PHOTO OF HELPER T CELL ACTIVATION
In the lymph nodes, helper T cells quickly scan dendritic cells to see if their cognate antigen is being displayed. A single dendritic cell typically hosts about 1,000 such "visits" each hour. If a T cell does find a dendritic cell displaying its cognate antigen, the T cell "lingers," because complete activation of a naive helper T cell usually takes several hours. During this time, a number of important events take place. First, adhesion molecules on the surface of the dendritic cell bind to their adhesion partners on the T cell, helping to keep the two cells together. Next, the CD4 co-receptor molecules on the surface of the T cell clip onto the class II MHC molecules on the dendritic cell and strengthen the interaction between the two cells. In addition, the engagement of its TCRs upregulates the expression of adhesion molecules on the Th cell surface, strengthening the "glue" that holds the APC and the T cell together. This is important, because the initial binding between a TCR and an MHC–peptide complex is actually rather weak – to allow for rapid scanning. Consequently, the Velcro-like adhesion molecules are extremely important for T cell activation. The clustering of TCRs and adhesion molecules at the point of contact between an APC and a T cell results in the formation of what immunologists call an immunological synapse.
Engagement of a helper T cell's receptors also upregulates expression of CD40L proteins on its surface, and when these proteins plug into the CD40 proteins on the surface of a dendritic cell, several remarkable things happen. Although mature dendritic cells express MHC and co-stimulatory molecules (e.g., B7) when they first enter lymph nodes, the expression level of these proteins increases when CD40 proteins on the APC are engaged by the CD40L proteins on a Th cell. In addition, engagement of a dendritic cell's CD40 proteins prolongs the life of the dendritic cell. This extension of a "useful" dendritic cell's life span makes perfect sense. It insures that the particular dendritic cells which are presenting a T cell's cognate antigen will stick around long enough to help activate a lot of these T cells. So the interaction between a dendritic cell and a naive helper T cell is not just one-way. These cells actually perform an activation "dance" in which they stimulate each other. The end result of this cooperation is that the dendritic cell becomes a more potent antigen presenting cell, and the Th cell is activated to express the high levels of CD40L required for helping activate B cells.
After activation is complete, the helper T cell and the antigen presenting cell part. The APC then goes on to activate other T cells, while the recently activated Th cells proliferate to build up their numbers. During an infection, a single activated T cell can give rise to about 10,000 daughter cells during the first week or so of proliferation. This proliferation is driven by growth factors such as IL-2. Naive T cells can make some IL-2, but they don't have IL-2 receptors on their surface – so they can't respond to this cytokine. In contrast, activated Th cells produce large amounts of IL-2, and they also express receptors for this cytokine on their surface. As a result, newly activated helper T cells stimulate their own proliferation. This coupling of activation to the upregulation of growth factor receptors is the essence of clonal selection: Those Th cells which are selected for activation (because their TCRs recognize an invader) upregulate their growth factor receptors and proliferate to form a clone.
So the sequence of events during the activation of a helper T cell is this: Adhesion molecules mediate weak binding between the Th and the APC while TCRs engage their cognate antigen presented by the APC. Receptor engagement strengthens the adhesion between the two cells, and upregulates CD40L expression on the Th cell. CD40L then binds to CD40 on the APC and stimulates the expression of MHC and co-stimulatory molecules (e.g., B7) on the APC surface. The co-stimulation provided by the APC amplifies the "TCR engaged" signal, making it easier to activate the Th cell. When activation is complete, the cells disengage, and the Th cell proliferates, driven by growth factors which bind to receptors that appear on the Th cell surface as a result of activation. This proliferation produces a clone of helper T cells which can recognize the invader advertised by the antigen presenting cell.
HOW KILLER T CELLS ARE ACTIVATED
For a helper T cell to be activated, its receptors must recognize their cognate antigen displayed by class II MHC molecules on the surface of an activated dendritic cell, and the Th cell must also receive co-stimulatory signals from that same dendritic cell. This requirement that two cells (the Th cell and the DC) agree that there has been an invasion is a powerful safeguard against the activation of "rogue" helper T cells – cells which might direct an attack against our own tissues, causing autoimmune disease.
Although the events involved in the activation of helper T cells are pretty clear, the picture of how naive killer T cells are activated is still rather fuzzy. Until recently, it was believed that for a naive killer T cell to be activated, three cells needed to be involved: a CTL with receptors that recognized the invader; an activated dendritic cell, which was using its class I MHC molecules to present fragments of the invader's proteins to the CTL; and an activated helper T cell which was providing "help" to the CTL. One way this might happen would be for the dendritic cell, the Th cell, and the CTL to engage in a ménage à trois. There is, however, a potential problem with this scenario. Early in an infection, there are very few of any of these cells around. Consequently, the probability is quite small that a helper T cell and a killer T cell would simultaneously find a dendritic cell which is presenting their cognate antigens.
Experiments have now shown that in response to an invasion by microbes which can infect cells (the microbes that CTLs are designed to defend against), T cell help is not required during the initial activation of killer T cells. A two-cell interaction between a naive CTL and an activated dendritic cell is sufficient. During this meeting, the CTL's receptors recognize their cognate antigen displayed by class I MHC molecules on the dendritic cell, and they receive co-stimulation from that same dendritic cell. What this means is that a naive killer T cell can be activated in a way that is analogous to the way a naive Th cell is activated: by encountering an activated dendritic cell.
Requiring only a two-cell interaction for the activation of naive Th cells and CTLs makes perfect sense in terms of getting the adaptive immune system fired up before invaders take over completely. However, although CTLs activated without Th cell help do proliferate somewhat to build up their numbers and can kill infected cells, these "helpless" CTLs do not kill with high efficiency, and they do not live very long. Consequently, helpless activation of CTLs results in a small "burst" of killer T cells designed to deal quickly with invaders early in an infection. In contrast, CTLs that are fully activated with assistance from helper T cells can proliferate robustly, can kill efficiently, and can become memory killer T cells (cells which can defend against a subsequent invasion by the same attacker). And this, of course, brings us back to the question of how CTLs can be fully activated by Th cells and DCs without requiring a three-cell interaction.
One possibility, the "sequential model," postulates that when helper T cells are activated, the dendritic cells which activate them become "licensed." Once licensed, these dendritic cells are presumed to be able to express class I MHC molecules, cytokines, and surface molecules that can fully activate CTLs which subsequently visit them. This sequence of events, in which the DC and the Th cell meet first, and then the licensed DC and the CTL meet later, would avoid the need for all three cells to meet simultaneously.
It also has been demonstrated that when an activated dendritic cell and a helper T cell "hook up," chemokines are generated which can attract naive killer T cells to their location, making a ménage à trois more likely. Moreover, the meeting between an activated dendritic cell and a helper T cell typically lasts for several hours. Consequently, cytokine-directed migration and extended Th–DC interaction times could give that rare CTL which also recognizes the invader a better chance to join the party. Finally, relatively late in an immune response, there will be many activated dendritic cells, Th cells, and killer T cells present in lymph nodes and other secondary lymphoid organs – perhaps enough of each of these cell types to make a three-cell interaction probable. My guess is that killer T cells can be activated by all of these mechanisms. But stay tuned!
FAIL-SAFE ACTIVATION
For either a naive helper T cell or a virgin CTL to be activated, the T cell must first recognize its cognate antigen presented by an antigen presenting cell. This is true even for helper T cell-independent activation of CTLs. As a consequence of this requirement for antigen presentation during activation, a fail-safe system is set up in which the decision to activate a T cell always involves more than one cell. This helps insure that the powerful weapons of the adaptive immune system only come into play when there is a genuine threat, and makes it less likely that a T cell will turn its weapons inward on its host.
REVIEW
There are many similarities between the ways B cells and T cells are activated. BCRs and TCRs both have "recognition" proteins that extend outside the cell and that are incredibly diverse because they are made by mixing and matching gene segments. For the BCR, these recognition proteins are the light and heavy chains that make up the antibody molecule. For the TCR, the molecules that recognize antigen are the α and β proteins. TCRs and BCRs have cytoplasmic tails that are too short to signal recognition, so additional molecules are required for this purpose. For the BCR, these signaling proteins are called Igα and Igβ. For the TCR, signaling involves a complex of proteins called CD3.
For B or T cells to be activated, their receptors must be clustered by antigen, because this crosslinking brings together many of their signaling molecules in a small region of the cell. When the density of signaling molecules is great enough, an enzymatic chain reaction is set off that conveys the "receptor engaged" signal to the cell's nucleus. There, in the "brain center" of the cell, genes involved in activation are turned off or on as a result of this signal.
Although crosslinking of receptors is essential for the activation of B or T cells, it is not enough. Naive B and T cells also require co-stimulatory signals that are not antigen specific. This two-signal requirement for activation sets up a fail-safe system which protects against the inappropriate activation of B or T cells. For B cell activation, a helper T cell can provide co-stimulation through surface proteins called CD40L that plug into CD40 proteins on the B cell surface. B cells also can be co-stimulated by "danger signals," including invader-specific molecular signatures or battle cytokines. For T cells, co-stimulation usually involves B7 proteins on an activated dendritic cell that engage CD28 proteins on the surface of the T cell.
Early in an infection, B cells and killer T cells can be activated without the assistance of helper T cells. Helpless plasma B cells make IgM antibodies because they have not switched to a class of antibodies that might be more appropriate to defend against the particular invader. They also have not undergone somatic hypermutation, so their BCRs have not been "fi ne tuned." And they only live a short time. Likewise, helpless CTLs do not proliferate robustly, are short-lived, and do not kill as efficiently as T cells which have been assisted by helper T cells. Although helpless B and T cells have these deficiencies, they can provide an important rapid response to pathogens while more "sophisticated" B and T cells are being produced.
Both BCRs and TCRs can associate with co-receptor molecules which serve to amplify the signal that the BCRs and TCRs send. For B cells, this co-receptor recognizes antigen that has been opsonized by complement. If the BCR recognizes an antigen, and if that antigen also is "decorated" with complement protein fragments, the antigen serves as a "clamp" that brings the BCR and the complement receptor together on the surface of the B cell, greatly amplifying the "receptor engaged" signal. As a consequence, B cells are much more easily activated (many fewer BCRs need to be crosslinked) by antigen that has been opsonized by complement.
T cells also have co-receptors. Th cells express CD4 co-receptor molecules on their surface, and CTLs express CD8 co-receptors. When a TCR binds to antigen presented by an MHC molecule, the co-receptor on the T cell surface clips onto the MHC molecule. This serves to strengthen the signal that is sent by the TCR to the nucleus, so that the T cell is more easily activated (fewer TCRs need to be crosslinked). These co-receptors only work with the "right" MHC types: class I for CTLs with CD8 co-receptors, and class II for Th cells with CD4 co-receptors. Consequently, co-receptors really are "focus" molecules. The B cell co-receptor helps B cells focus on antigens that have already been identified by the complement system as dangerous (those that have been opsonized). The CD4 co-receptor focuses the attention of Th cells on antigens displayed by class II MHC molecules, and the CD8 co-receptor focuses CTLs on antigens displayed by class I MHC molecules.
Of course, there is an important difference between what B cells and T cells "look at." The BCR recognizes antigen in its "natural" state – that is, antigen which has not been chopped up and bound to MHC molecules. This antigen can be a protein or almost any other organic molecule (e.g., a carbohydrate or a fat). In contrast, the αβ receptors of traditional T cells only recognize fragments of proteins presented by classical MHC molecules. And whereas a B cell's receptors only bind to one thing – its cognate antigen – the TCR binds to both the presented peptide and the MHC molecule. Because the universe of antigens recognized by the BCR includes proteins, carbohydrates, and fats, B cells can respond to a greater variety of invaders than can T cells. On the other hand, because the TCR looks at small fragments of proteins, it can recognize targets that are hidden from view of the BCR in an intact and tightly folded protein.
Another difference between B cells and T cells is that during an infection, the BCR can undergo somatic hyper-mutation and selection. So B cells can "draw from the deck" to try to get a better hand. In contrast, the TCR does not hypermutate, so T cells must be satisfied with the cards they are dealt.


