第6讲 T Cells at Work
HEADS UP!
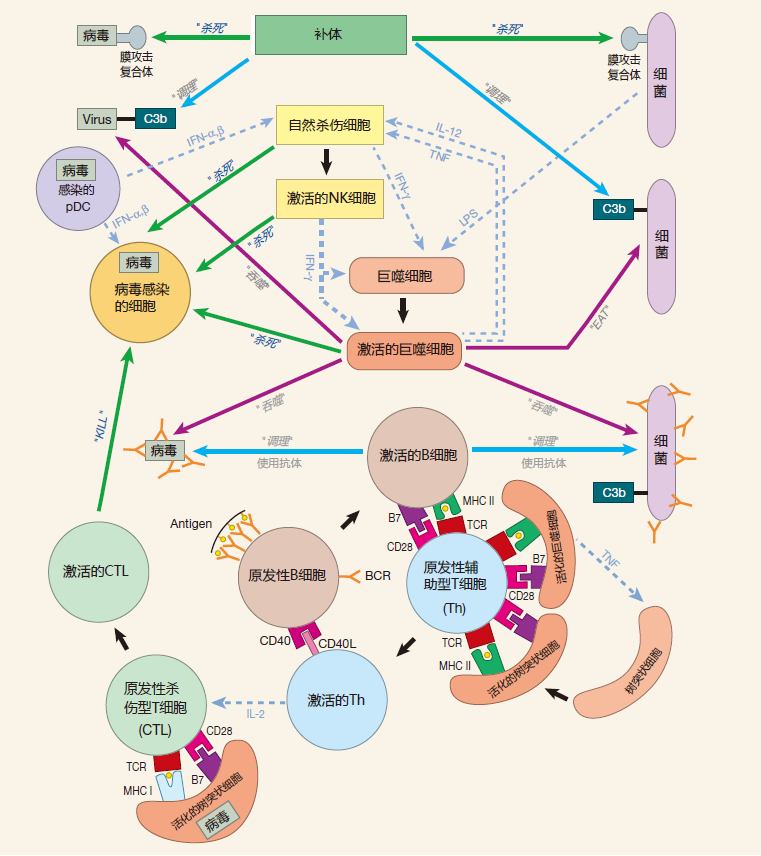
Two of the most important weapons of the adaptive immune system are helper T cells, which secrete just the right combination of cytokines to orchestrate an appropriate defense, and killer T cells, which can "execute" infected cells and the pathogens within them. However, it is the innate immune system which "instructs" the adaptive immune system, telling it which weapons to mobilize to defend against a given invader and where these weapons should be deployed in the body.
INTRODUCTION
Once helper T cells and killer T cells have been activated, they are ready to go to work – to become what immunologists call effector cells. The primary job of an effector CTL is to kill cells that have been infected by viruses or bacteria. Effector helper T cells have two main duties: They can remain in the blood and lymphatic circulation and travel from lymph node to lymph node, providing help for B cells or for killer T cells, or they can exit blood vessels at the sites where a battle is going on to provide help for the soldiers of the innate and adaptive immune systems.
HELPER T CELLS AS CYTOKINE FACTORIES
Helper T cells can produce many different cytokines – protein molecules which they use to communicate with the rest of the immune system. As the "quarterback" of the immune system team, the helper T cell uses cytokines to "call the plays." These include cytokines such as TNF, IFN-γ, IL-4, IL-5, IL-6, IL-10, IL-17, and IL-21. However, a single Th cell doesn't secrete all these different cytokines. In fact, Th cells tend to secrete subsets of cytokines – subsets which are appropriate to orchestrate an immune defense against particular invaders. So far, three major subsets have been identified: Th1, Th2, and Th17. You shouldn't take this to mean, however, that there are only three different combinations of cytokines that can be secreted by Th cells.
In fact, immunologists initially had a hard time finding helper T cells that secreted exactly the Th1 or Th2 cytokine subsets in humans. Clearly, there are helper T cells which give off mixtures of cytokines that don't conform to the Th1/Th2/Th17 paradigm. Nevertheless, this concept turns out to be quite useful in trying to make sense of the combinations of cytokines (the cytokine "profiles") that Th cells produce. I also should mention that in addition to these three Th subsets which are involved in activating the immune system, there is a subset of Th cells which functions to suppress the immune response. We will discuss those "Treg" cells in subsequent lectures.
Of course, all of this begs the question: How does a helper T cell know which cytokines are appropriate for a given situation? Well, as any football fan knows, behind every good quarterback, there is a good coach.
THE DENDRITIC CELL AS "COACH" OF THE IMMUNE SYSTEM TEAM
For a helper T cell to make an informed decision about which cytokines to make, at least two pieces of information are required. First, it's necessary to know what type of invader the immune system is dealing with. Is it a virus, a bacterium, a parasite, or a fungus? Second, it is essential to determine where in the body the invaders are located.
Are they in the respiratory tract, the digestive tract, or the big toe? Virgin helper T cells don't have direct access to either type of information. After all, they are busy circulating through the blood and lymph, trying to find their cognate antigen. What is needed is an "observer" who has actually been at the battle site, who has collected the pertinent information, and who can pass it along to the helper T cell. And which of the immune system cells could qualify as such an observer? The dendritic antigen presenting cell, of course!
Just as a football coach scouts the opposing team and formulates a game plan, so a dendritic cell, acting as the "coach" of the immune system team, collects information on the invasion, and decides how the immune system should react. That's why dendritic cells are so important.
They don't just turn naive helper T cells and killer T cells on. Dendritic cells actually function as the "brains" of the immune system, processing information pertaining to the invasion and producing a plan of action.
What are the inputs that dendritic cells integrate to produce the game plan? These are of two types. The first input comes to the dendritic cell through the pattern-recognition receptors we discussed in Lecture 2. These cellular receptors recognize conserved patterns that are characteristic of various classes of invaders. For example, Toll-like receptor 4 (TLR4) senses the presence of LPS, which is component of the outer cell membrane of Gram-negative bacteria. TLR4 also can detect proteins made by certain viruses. TLR2 specializes in identifying molecules that are "signatures" of Gram-positive bacteria. TLR3 recognizes the double-stranded RNA produced during many viral infections. And TLR9 recognizes the unmethylated DNA dinucleotide, CpG, which is characteristic of bacterial DNA.
Although TLRs were the first pattern-recognition receptors to be characterized, additional families of pattern-recognition receptors have now been discovered. Consequently, the emerging picture is that different types of antigen presenting cells (e.g., dendritic cells or macrophages) in different parts of the body display distinct sets of these pattern-recognition receptors which are "tuned" to recognize various structural features of common microbial invaders. By integrating the signals from these diverse pattern-recognition receptors, an APC gathers information on the type of invader to be defended against.
The second "scouting report" dendritic cells employ when formulating their game plan is received through various cytokine receptors on their surface. Because different pathogens elicit the production of different cytokines during an infection, dendritic cells can learn a lot about an invader by sensing the cytokine environment. So dendritic cells out on the front lines collect "intelligence" about an invader through pattern-recognition receptors and cytokine receptors. It is then up to the dendritic cell to "decode" these inputs in order to discern the type of invasion, and to decide which weapons need to be mobilized.
"Ordinary" cells in different areas of the body (e.g., skin cells or cells that underlie the intestines) produce characteristic mixtures of cytokines in response to invaders, and these cytokines provide dendritic cells with information about the area of the body that is under attack. In fact, these cytokines imprint dendritic cells with a "regional identity." This ability to remember their "roots" helps DCs dispatch the weapons of the adaptive immune system to the parts of the body where they are needed.
But how is the dendritic cell's game plan conveyed to the Th cell – the cell that will direct the action? There are two ways that the coach instructs the quarterback. First, the mixture of co-stimulatory molecules displayed on the surface of an activated dendritic cell will depend on the type of invader the DC has encountered. These co-stimulatory molecules can "plug into" receptor molecules on the surface of helper T cells to pass this information along.
Although B7 is the best-studied co-stimulatory molecule, other co-stimulatory molecules have been identified, and more are certain to be discovered.
In addition to co-stimulatory surface molecules, activated dendritic cells produce cytokines which can convey information to the helper T cell. So the bottom line is this: Co-stimulatory molecules and cytokines are used by dendritic cells to pass along the "game plan" to helper T cells. And the particular combination of co-stimulatory molecules and cytokines which a dendritic cell offers to a Th cell will depend on what the dendritic cell has observed at the battle scene. To get a better idea of how this all works, let's look more closely at the Th1, Th2, and Th17 subsets of cytokines.
Th1 HELPER T CELLS
If you have a puncture wound that results in a bacterial infection or if you are attacked by a virus that replicates in the tissues, resident dendritic cells will be alerted through their pattern-recognition receptors and by receiving battle cytokines produced by macrophages and other cells in the inflamed tissues. These signals activate the dendritic cell and imprint it with the special characteristics of an APC which has observed a bacterial or viral infection in the tissues. The details of exactly how this is accomplished aren't clear yet, but the result is that when this DC leaves such a battle site and travels through the lymph to a nearby lymph node, it will produce the cytokine IL-12. And when the IL-12-producing DC presents the battle antigens it has acquired to a virgin helper T cell, that Th cell will be instructed to become a helper T cell which produces the "classical" Th1 cytokines: TNF, IFN-γ, and IL-2.
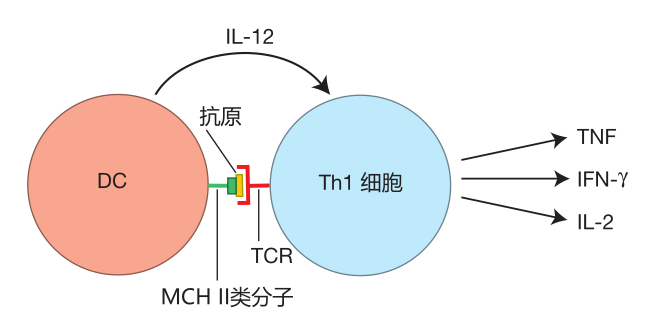
Why these particular cytokines? Let's see what these cytokines do. The TNF secreted by Th1 helper T cells helps activate macrophages and natural killer cells.
However, macrophages only stay activated for a limited time. They are lazy fellows which like to go back to resting and garbage collecting. Fortunately, the IFN-γ produced by Th1 cells acts as a "prod" that keeps macrophages fired up and engaged in the battle. IFN-γ also influences B cells during class switching to produce human IgG3 antibodies. These antibodies are especially good at opsonizing viruses and bacteria and at fixing complement.
NK cells can kill three or four target cells in about 16 hours, but then they "tire out." The IL-2 produced by Th1 cells can "recharge" NK cells, enabling them to continue killing. In addition, IL-2 is a growth factor which stimulates the proliferation of CTLs, NK cells, and Th1 cells themselves – so that more of these important weapons will be available to deal with the attack.
Altogether, the Th1 cytokines are the perfect package to help defend against a viral or bacterial attack in the tissues. The Th1 cytokines instruct the innate and adaptive systems to mobilize cells and produce antibodies that are especially effective against these invaders, and these cytokines also keep the warriors of the immune system fired up until the invaders have been defeated.
Th2 HELPER T CELLS
Now suppose that you have been infected by a parasite (e.g., hook worms) or you have eaten some food that is contaminated with pathogenic bacteria. In the tissues that line your intestines, a battle will be raging. Dendritic cells from that area will travel to nearby lymph nodes, and will activate those helper T cells which have T cell receptors that can recognize the worm or bacterial antigens presented by the DC. This results in helper T cells that are "programmed" to produce the Th2 subset of cytokines, which includes IL-4, IL-5, and IL-13.
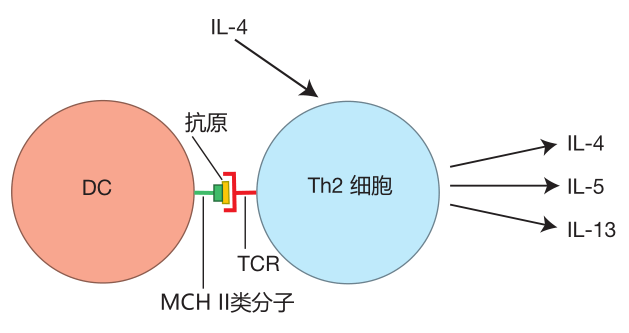
Why IL-4, IL-5, and IL-13, you ask? IL-4 is a growth factor that stimulates the proliferation of helper T cells which have committed to secrete the Th2 profile of cytokines. So, like Th1 cells, Th2 cells produce their own growth factor.
IL-4 also is a growth factor for B cells, and this cytokine can influence B cells to class switch to produce IgE antibodies – powerful weapons against parasites such as hook worms. IL-5 is a cytokine which encourages B cells to produce IgA antibodies, antibodies that are especially useful against bacteria which invade via the digestive tract. And IL-13 stimulates the production of mucus in the intestines. This mucus helps prevent more intestinal parasites or pathogenic bacteria from breaching the intestinal barrier and entering the tissues. So the Th2 cytokine profile is just the ticket if you need to defend against parasites or pathogenic bacteria that have invaded via the digestive tract.
In the figure above, you will notice that IL-4, which causes a naive Th cell to commit to becoming a Th2 cell, does not come from the dendritic cell. Of course, once the helper T cell commits to the Th2 cytokine profile, there will be plenty of IL-4 around – because this is one of the cytokines Th2 cells secrete. Nevertheless, the source of IL-4 initially required for Th2 commitment has not yet been identified.
Th17 HELPER T CELLS
If areas of the body protected by mucosal barriers are attacked by fungi (e.g., a vaginal yeast infection) or by extracellular bacteria, DCs will travel to a nearby lymph node to activate helper T cells which recognize the antigens the DC is presenting. These traveling dendritic cells can produce TGFβ and either IL-6 or IL-23, which, together with co-stimulatory molecules, will influence newly activated helper T cells to produce the Th17 subset of cytokines, which includes IL-17, IL-21, and IL-23.
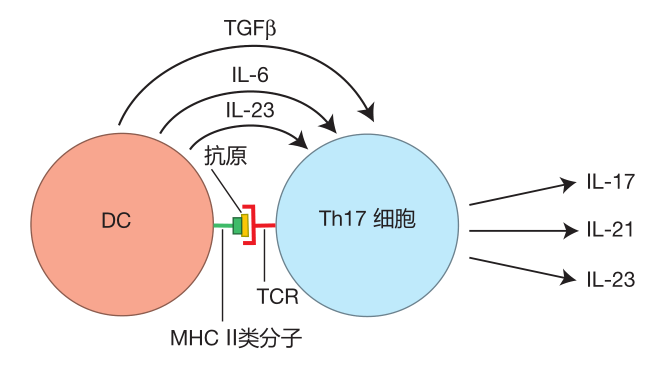
Secretion of the "signature cytokine," IL-17, results in the recruitment of massive numbers of neutrophils to the site of infection. These neutrophils help defend against fungi and some extracellular bacteria against which warriors recruited by Th1 and Th2 cells would be relatively ineffective. Indeed, patients who have a genetic defect in IL-17 secretion suffer from devastating fungal infections (e.g., infection with the common yeast, Candida albicans) even though their Th1 and Th2 helper T cells function normally. IL-23 is a growth factor which causes helper T cells that have committed to being Th17 cells to proliferate to build up their numbers. IL-21 can cause B cells that guard the mucosal surfaces to produce IgG3 and IgA antibodies. IgG3 is an antibody isotype that is especially good at activating the complement cascade on the surface of bacteria, and IgA antibodies can bind invaders and help usher them out of the body with the mucus. So if you are attacked by fungi or extracellular bacteria, the cytokines secreted by Th17 cells are there to help protect you.
Th0 HELPER T CELLS
Some helper T cells (the Th0 cells) remain "unbiased" when they first are activated, retaining the ability to produce a wide range of cytokines. It appears that DCs tell these helper T cells where to go, but not what to do. However, once Th0 cells reach the battle scene, the cytokine environment they encounter there causes them to commit to the cytokine profile required for the defense. For example, when Th0 cells exit the blood to fight a bacterial infection in the tissues, they encounter an environment rich in IL-12. This is because Th1 cells that are already fighting bacteria there produce IFN-γ. This cytokine, together with danger signals such as the bacterial molecule LPS, activates tissue macrophages, which secrete large amounts of IL-12. And when Th0 cells receive the IL-12 signal, they "realize" what type of battle is being fought, and commit to becoming Th1 cells – Th cells which produce the cytokines needed to defend against bacteria.
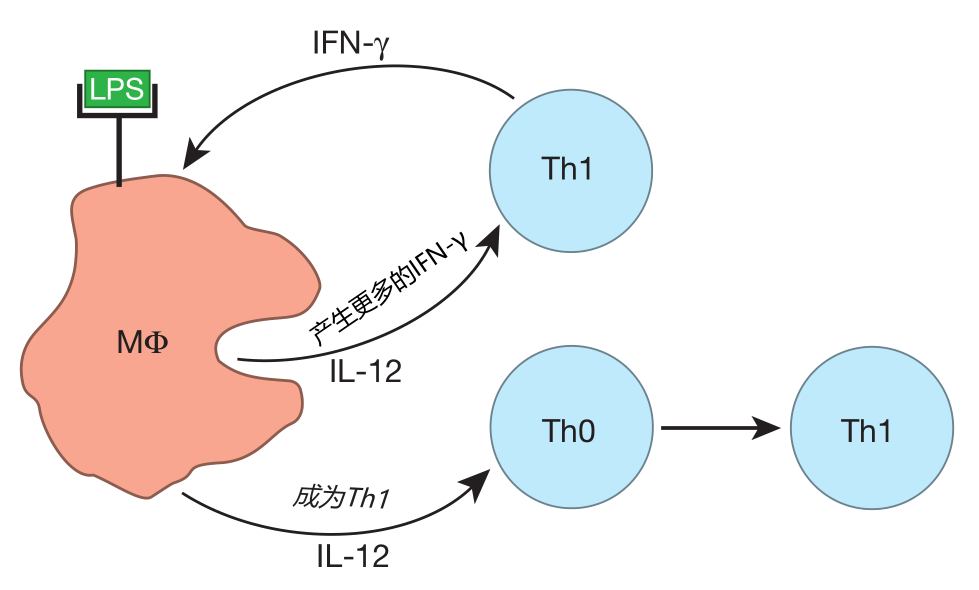
Likewise, Th0 cells can become Th2 or Th17 cells when they reach a battle site that is rich in IL-4 or IL-6 and TGFβ, respectively. So previously uncommitted Th0 cells can be "converted" by the cytokine environment at the scene of the battle to become Th1, Th2, or Th17 cells.
LOCKING IN THE HELPER T CELL PROFILE
Once helper T cells commit to a particular cytokine profile, they begin to secrete cytokines which encourage the proliferation of that particular type of Th cell – be it Th1, Th2, or Th17. This sets up a positive feedback loop which results in even more of the "selected" Th cells being produced.
In addition to positive feedback, there is also negative feedback at work. For example, IFN-γ made by Th1 cells actually decreases the rate of proliferation of Th2 cells, so that fewer Th2 cells will be produced. And one of the Th2 cytokines, IL-10, acts to decrease the rate of proliferation of Th1 cells. The result of all this positive and negative feedback is a large number of helper T cells which are strongly biased toward the production of a certain subset of cytokines.
There is an important point about helper T cell bias which I want to be sure you understand. Cytokines have a very limited range. They can travel only short distances in the body before they are captured by cellular receptors or are degraded. Consequently, when we talk about helper T cells being biased toward secreting a certain cytokine profile, we are talking about something very local. Clearly, you wouldn't want every Th cell in your body to be of the Th1 type, because then you'd have no way to defend against a respiratory infection. Conversely, you wouldn't want to have only Th2 cells, because the IgA or IgE antibodies made in response to the Th2 cytokines would be useless if you get a bacterial infection in your big toe. In fact, it is the local nature of cytokine signaling which gives the immune system the flexibility to simultaneously mount defenses against many different invaders that threaten different parts of the body.
It is also important to note that dendritic cells are members of the innate system team. Consequently, the innate immune system not only informs the adaptive system when there is danger, it also "coaches" the adaptive system to insure that the appropriate weapons are sent to the right places.
DELAYED-TYPE HYPERSENSITIVITY
There is an example of "signal calling" by Th cells that I think you'll find interesting. It is termed delayed-type hypersensitivity (DTH), and it was first observed by Robert Koch when he was studying tuberculosis back in the latter part of the nineteenth century. Koch purified a protein, tuberculin, from the bacterium which causes tuberculosis, and used this protein to devise his famous "tuberculin skin test." If you've had this test, you'll recall that a nurse injected something under your skin and told you to check that area in a few days. If the spot where you were injected became red and swollen, you were instructed to come back in to see the doctor. Here's what that's all about.
The "something" you were injected with was Koch's tuberculin protein. If you have active TB or have been infected with it in the past, your immune system will include memory Th1 cells that were made in response to the infection. When the nurse injects the tuberculin protein, dendritic cells stationed beneath the skin take up the protein and present tuberculin peptides to these memory cells – and they are reactivated. Now the fun begins, because these Th cells secrete IFN-γ and TNF – Th1-type cytokines that activate resident tissue macrophages near the site of injection and help recruit neutrophils and additional macrophages to the area. The result is a local inflammatory reaction with redness and swelling: the signal that your TB test is positive. Of course, the reason you have to wait several days for the test to "develop" is that memory helper T cells must be reactivated, proliferate, and produce those all-important cytokines that orchestrate the inflammatory reaction.
On the other hand, if you have never been exposed to the tuberculosis bacterium, you will have no memory helper T cells to reactivate. Without the cytokines supplied by activated Th cells, there will be no inflammatory reaction to the tuberculin protein, and your skin test will be scored as negative.
What is interesting here is that delayed-type hypersensitivity is both specific and non-specific. The specificity comes from Th cells that direct the immune response after recognizing the tuberculin peptide presented by dendritic cells. The non-specific part of the reaction involves the neutrophils and macrophages that are recruited and activated by cytokines secreted by the Th cells. This is yet another example of the cooperation that goes on between the adaptive and innate immune systems.
You may be wondering why the tuberculin used for the test doesn't activate naive T cells, so that the next time you are tested, you will get a positive reaction. The reason is that the tuberculin protein does not, by itself, cause an inflammatory reaction (i.e., a battle situation), and you remember that dendritic cells only mature and carry antigen to a lymph node if a battle is on. Consequently, if a protein that is injected under your skin is judged by the innate system to be not dangerous, the adaptive immune system will not be activated. This illustrates again how important the innate immune system is for initiating an immune response: If your innate system does not recognize an invader as dangerous and put up a fight, your adaptive system usually will just ignore the intrusion.
HOW CTLs KILL
So far in this lecture, we have discussed what activated helper T cells do. Now it is time to focus on killer T cells.
Once a CTL has been activated, it proliferates rapidly to build up its numbers. These effector T cells then leave the lymph node, enter the blood, and travel to the area of the body where the invaders they can kill are located. When an effector T cell reaches the battle site, it exits the blood, and begins to hack away at infected cells. Most killing by CTLs requires contact between the CTL and its target cell, and CTLs have several weapons they can use during this "hand-to-hand" combat.
One weapon CTLs employ involves the production of a protein called perforin. Perforin is a close relative of the C9 complement protein that is part of the membrane attack complex. Like its cousin, perforin can bind to cell membranes and drill holes in them. For this to happen, a killer T cell's TCRs must first identify the target. Then adhesion molecules on the CTL hold the target cell close while the killer cell delivers a mixture of perforin and an enzyme called granzyme B onto the surface of the target cell. What happens next is still a bit uncertain, but the latest thinking is this: The perforin damages the target cell's outer membrane, and when the cell tries to repair this damage, both granzyme B and perforin are taken into the cell in a vesicle made from the target cell's membrane. Once inside the target cell, the perforin molecules make holes in the entry vesicle, allowing the granzyme B to escape into the cytoplasm of the cell. So perforin helps a CTL deliver granzyme B into the cytoplasm of its target cell where granzyme B triggers an enzymatic chain reaction that causes the cell to commit suicide by apoptosis. This kind of "assisted suicide" usually involves the destruction of the target cell's DNA by the cell's own enzymes. One important feature of this type of killing is that it is "directed": The CTL delivers its lethal cargo right onto the target cell, so that other cells in the area are not damaged during the slaughter. After a killer T cell has made contact with its target, it only takes about half an hour to kill the cell, and during each attack, the CTL only uses a fraction of its perforin and granzyme B. Consequently, a single killer T cell can execute multiple target cells. You may be wondering why the CTL doesn't kill itself when it delivers these deadly enzymes to the surface of its target. Nobody knows!
The second way a CTL can kill is by using a protein on its surface called Fas ligand (FasL) which can bind to the Fas protein on the surface of a target cell. When this happens, a suicide program is set in motion within the target cell, and, again, the cell dies by apoptosis.
Interestingly, natural killer cells use these same two mechanisms (perforin/granzyme B or FasL) to kill their targets.
It is worth mentioning here that there actually are two different ways a cell can die: by necrosis or by apoptosis.
Although the end result is the same (a dead cell), the two processes are quite different. Cells usually die by necrosis either as the result of a wound (e.g., a cut or a burn) or when they are killed by an attacking virus or bacterium.
During necrosis, enzymes and chemicals that normally are safely contained within a living cell are released by the dying cell into the surrounding tissues, where they can do real damage. In contrast, death by apoptosis is much tidier. As a cell dies by apoptosis, its contents are enclosed in little "garbage bags" (vesicles) made from the outer membrane of the dying cell. These vesicles are then eaten and destroyed by nearby macrophages as part of their garbage collecting duty. Consequently, during apoptosis, the contents of the target cell don't get out
into the tissues to cause damage. So by killing their targets by inducing apoptosis rather than necrosis, CTLs can rid the body of virus-infected cells without causing the collateral tissue damage that would result from necrotic cell death.
There is another reason why triggering cells to die by apoptosis is an especially effective way for killer T cells to destroy virus-infected cells. When virus-infected cells die by apoptosis, the DNA of unassembled viruses is destroyed along with the target cell's DNA. In addition, DNA or RNA viruses that have reached various stages of assembly within the cell are enclosed in apoptotic vesicles and are disposed of by macrophages. It is this ability to destroy infected cells and the viruses they contain by inducing apoptosis that makes a killer T cell such a potent antiviral weapon.
Although the main job of CTLs is to destroy infected cells, killer T cells also can secrete cytokines. For example, CTLs can produce IFN-γ, a cytokine that upregulates expression of class I MHC molecules on nearby cells. This results in a more robust class I display, making it easier for CTLs to recognize infected cells.
REVIEW
In your body, dendritic antigen presenting cells are stationed beneath all surfaces that are exposed to the outside world. Because of where they are located, DCs can observe an invasion first hand. In fact, the intelligence they acquire at the scene of the battle is complete enough to allow them to formulate a plan of action for the rest of the immune system. This information is gathered in part through the dendritic cell's pattern-recognition receptors, which detect the "signatures" of different types of invaders. In addition, dendritic cells have receptors which sense the cytokines given off by other immune system cells that are engaged in the battle. Non-immune cells which reside where the battle is raging also can produce cytokines, and these cytokines can imprint dendritic cells with a regional identity – so that they "remember" where the battle is taking place.
Armed with all this information on the type of invader and the location of the attack, dendritic cells travel to nearby lymph nodes, where they activate T cells. During this process, the game plan is conveyed to helper T cells in the form of co-stimulatory molecules and cytokines expressed by the dendritic cells. This information tells helper T cells which cytokines to make in order to orchestrate the appropriate defense against a particular invader.
In a sense, the dendritic cell functions as the coach of the immune system team, while the Th cell performs the duties of quarterback, calling the plays designed by the coach. The dendritic cell is part of the innate immune system. Consequently, the innate system not only determines when the adaptive system should be activated in response to danger, but it also instructs the adaptive system on which weapons to deploy and where to send them.
In response to the instructions delivered by dendritic cells, helper T cells produce combinations of cytokines that mobilize the weapons especially suited to deal with the invader which is attacking at the moment. Uncommitted Th cells also can be dispatched to the scene of the conflict where, under the influence of battle cytokines, they become committed to secreting a particular cytokine profile. And once a Th cytokine profile has been established, positive and negative feedback tend to lock in this particular profile. Importantly, the cytokines produced by helper T cells have a very short range, so their effects are quite "local." This feature allows the immune system to defend against different types of invaders which attack different parts of the body.
When we are attacked by viruses or bacteria which infect human cells, dendritic cells can activate killer T cells and dispatch them to the area of the body which is under attack. CTLs destroy infected cells by forcing them to commit suicide by a process called apoptosis. When a cell dies by apoptosis, its contents are enclosed in vesicles which are quickly ingested by nearby macrophages. This garbage disposal system keeps the potentially destructive chemicals and enzymes within the dying cell from getting out into the tissues and doing damage. And triggering cells to die by apoptosis has the great advantage that the pathogens which infected the cell also are packaged up and disposed of.