第7讲 Secondary Lymphoid Organs and Lymphocyte Trafficking
HEADS UP!
The secondary lymphoid organs are strategically placed to intercept invaders which penetrate our barrier defenses. During an infection, rare T cells must find antigen presenting cells which display their cognate antigen, and B cells must encounter the small number of helper T cells that can assist them in producing antibodies. Secondary lymphoid organs make it possible for antigen presenting cells, T cells, and B cells to meet under conditions that favor activation. The trafficking of immune system cells throughout our body is controlled by the modulated expression of adhesion molecules on the surface of these cells. Virgin and experienced lymphocytes move in different traffic patterns.
INTRODUCTION
In earlier lectures, we discussed the requirements for B and T cell activation. For example, in order for a helper T cell to assist a B cell in producing antibodies, that Th cells must first be activated by finding an antigen presenting cell which is displaying its cognate antigen. Then the B cell must find that same antigen displayed in a fashion which crosslinks its receptors. And finally, the B cell must find the activated Th cell. When you recognize that the volume of a T or B cell is only about one one hundred-trillionth of the volume of an average human, the magnitude of this "finding" problem becomes clear. Indeed, it begs the question, "How could a B cell ever be activated?"
The answer is that the movements of the various immune system players are carefully choreographed, not only to make activation efficient, but also to make sure that the appropriate weapons are delivered to the locations within the body where they are needed. Consequently, to really understand how this system works, one must have a clear picture of where in the body all these interactions take place. So it is time now for us to focus on the "geography"of the immune system.
The immune system's defense against an attacker actually has three phases: recognition of danger, production of weapons appropriate for the invader, and transport of these weapons to the site of attack. The recognition phase of the adaptive immune response takes place in the secondary lymphoid organs. These include the lymph nodes, the spleen, and the mucosal-associated lymphoid tissue (called the MALT for short). You may be wondering: If these are the secondary lymphoid organs, what are the primary ones? The primary lymphoid organs are the bone marrow, where B and T cells are born, and the thymus, where T cells receive their early training.
LYMPHOID FOLLICLES
All secondary lymphoid organs have one anatomical feature in common: They all contain lymphoid follicles. These follicles are critical for the functioning of the adaptive immune system, so we need to spend a little time getting familiar with them. Lymphoid follicles start life as "primary" lymphoid follicles: loose networks of follicular dendritic cells (FDCs) embedded in regions of the secondary lymphoid organs that are rich in B cells. So lymphoid follicles really are islands of follicular dendritic cells within a sea of B cells.
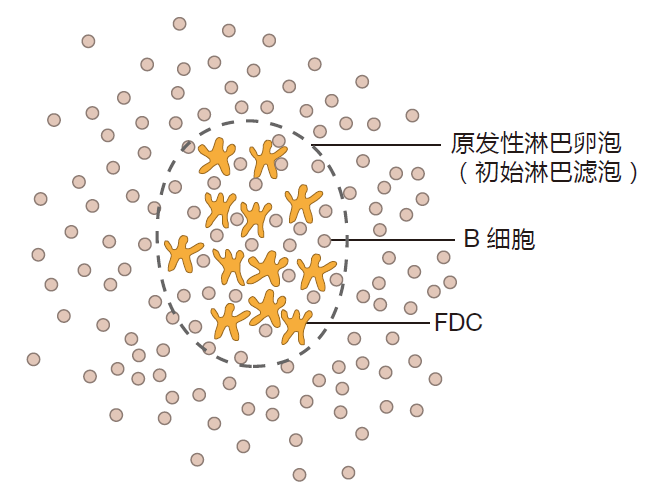
Although FDCs do have a starfish-like shape, they are very different from the antigen presenting dendritic cells (DCs) we talked about before. Those dendritic cells are white blood cells that are produced in the bone marrow, and which then migrate to their sentinel positions in the tissues. Follicular dendritic cells are regular old cells (such as skin cells or liver cells) that take up their final positions in the secondary lymphoid organs as the embryo develops. In fact, follicular dendritic cells are already in place during the second trimester of gestation. Not only are the origins of follicular dendritic cells and antigen presenting dendritic cells quite different, these two types of starfish-shaped cells have very different functions. Whereas the role of dendritic APCs is to present antigen to T cells via MHC molecules, the function of follicular dendritic cells is to display antigen to B cells. Here's how this works.
Early in an infection, complement proteins bind to invaders, and some of this complement-opsonized antigen will be delivered by the lymph or blood to the secondary lymphoid organs. Follicular dendritic cells that reside in these organs have receptors on their surface which bind complement fragments, and as a result, follicular dendritic cells pick up and retain complement-opsonized antigen. In this way, follicular dendritic cells become "decorated" with antigens that are derived from the battle being waged out in the tissues. Moreover, by capturing large numbers of antigens and by holding them close together, FDCs display antigens in a way that can crosslink B cell receptors. Later during the battle, when antibodies have been produced, invaders opsonized by antibodies also can be retained on the surface of follicular dendritic cells – because FDCs have receptors that can bind to the constant region of antibody molecules.
So follicular dendritic cells capture opsonized antigens and "advertise" these antigens to B cells in a configuration that can help activate them. Those B cells whose receptors are crosslinked by binding to their cognate antigens, hanging from these follicular dendritic "trees," proliferate to build up their numbers. And once this happens, the follicle begins to grow and become a center of B cell development. Such an active lymphoid follicle is called a "secondary lymphoid follicle" or germinal center. The role of complement-opsonized antigen in triggering the development of a germinal center cannot be overemphasized: Lymphoid follicles in humans who have a defective complement system never progress past the primary stage. Thus, we see again that for the adaptive immune system to respond, the innate system must first react to impending danger.
As they proliferate in germinal centers, B cells become very "fragile." Unless they receive the proper "rescue" signals, they will commit suicide (die by apoptosis). Fortunately, helper T cells can rescue these B cells by providing the co-stimulation they need. And when a B cell whose
receptors have been crosslinked by antigen receives the required co-stimulatory signals, it is temporarily rescued from apoptotic death, and continues to proliferate.
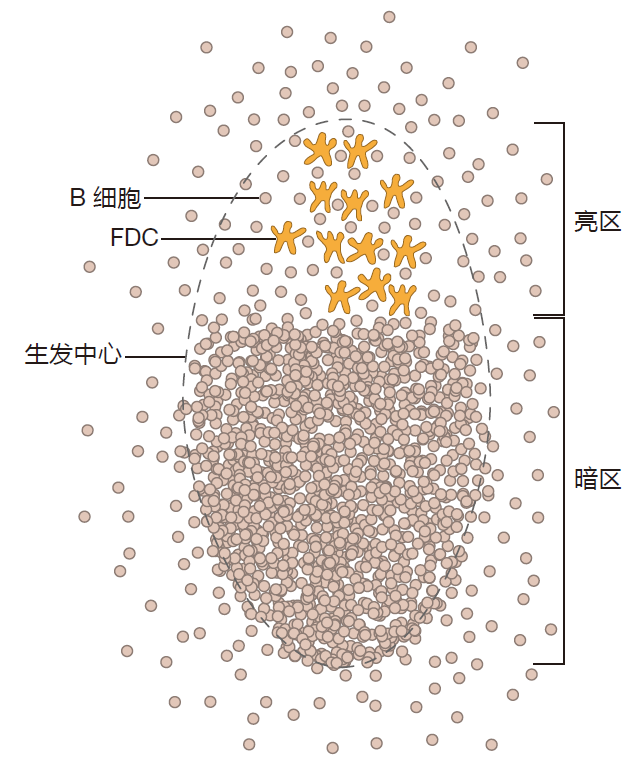
The rate at which B cells multiply in a germinal center is truly amazing: The number of B cells can double every six hours! These proliferating B cells push aside other B cells that have not been activated, and establish a region of the germinal center called the "dark zone" – because it contains so many proliferating B cells that it looks dark under a microscope.
After this period of proliferation, some of the B cells "choose" to become plasma B cells and leave the germinal center to produce antibodies. Because these B cells have received T cell help, they are fully competent to produce large quantities of invader-specific antibodies. However, these are mostly IgM antibodies because these B cells have not class switched. They also have not undergone somatic hypermutation to increase the average affinity of their receptors for their cognate antigen. Consequently, these B cells produce good antibodies – but not great antibodies. But that's okay. These IgM, "unrefined" antibodies are extremely useful early in infection before better antibodies can be made.
Other B cells remain in the germinal center, proliferate some more, and undergo somatic hypermutation to enhance the affinity of their receptors for antigen. Somatic hypermutation takes place in the dark zone of the germinal center, and after each round of hypermutation, the B cells migrate to the light zone where the affinity of their mutated BCRs for antigen is tested. Those B cells whose mutated BCRs do not have a high enough affinity for antigen will die by apoptosis, and will be eaten by macrophages in the germinal center. In contrast, B cells are rescued from apoptosis if the affinity of their receptors is great enough to be efficiently crosslinked by their cognate antigen displayed on FDCs – and if they also receive co-stimulation from activated Th cells that are present in the light zone of the germinal center. The picture is that B cells "cycle" between periods of proliferation and mutation in the dark zone and periods of testing and re-stimulation in the light zone. Sometime during all this action, probably in the dark zone, B cells can switch the class of antibody they produce.
In summary, lymphoid follicles are specialized regions of secondary lymphoid organs in which B cells percolate through a lattice of follicular dendritic cells that have captured opsonized antigen on their surface.
B cells that encounter their cognate antigen and receive T cell help are rescued from death. These "saved" B cells proliferate and can undergo somatic hypermutation and class switching. Clearly lymphoid follicles are extremely important for B cell development. That's why all secondary lymphoid organs have them.
HIGH ENDOTHELIAL VENULES
A second anatomical feature common to all secondary lymphoid organs except the spleen is the high endothelial venule (HEV). The reason HEVs are so important is that they are the "doorways" through which B and T cells enter these secondary lymphoid organs from the blood. Most endothelial cells that line the inside of blood vessels resemble overlapping shingles which are tightly "glued" to the cells adjacent to them to prevent the loss of blood cells into the tissues. In contrast, within most secondary lymphoid organs, the small blood vessels that collect blood from the capillary beds (the postcapillary venules) are lined with special endothelial cells that are shaped more like a column than like a shingle.
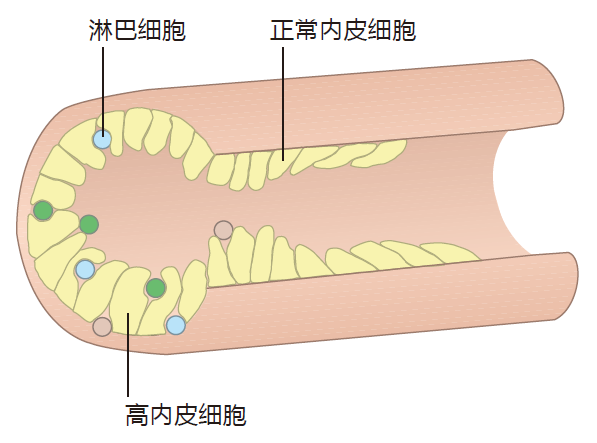
These tall cells are the high endothelial cells. So a high endothelial venule is a special region in a small blood vessel (venule) where there are high endothelial cells.
Instead of being glued together, high endothelial cells are "spot welded." As a result, there is enough space between the cells of the HEV for lymphocytes to wriggle through.
Actually, "wriggle" may not be quite the right term, because lymphocytes exit the blood very efficiently at these high endothelial venules: Each second, about 10,000 lymphocytes exit the blood and enter an average lymph node by passing between high endothelial cells.
Now that you are familiar with lymphoid follicles and high endothelial venules, we are ready to take a tour of some of the secondary lymphoid organs. On our tour today, we will visit a lymph node, a Peyer's patch (an example of the MALT), and the spleen. As we explore these organs, you will want to pay special attention to the "plumbing." How an organ is connected to the blood and lymphatic systems gives important clues about how it functions.
LYMPH NODES
A lymph node is a plumber's dream. This bean-shaped organ has incoming lymphatics which bring lymph into the node, and outgoing lymphatics through which lymph exits. In addition, there are small arteries (arterioles) carrying the blood that nourishes the cells of the lymph node as well as veins through which this blood leaves the node.
If you look carefully at this figure, you can also see the high endothelial venules.
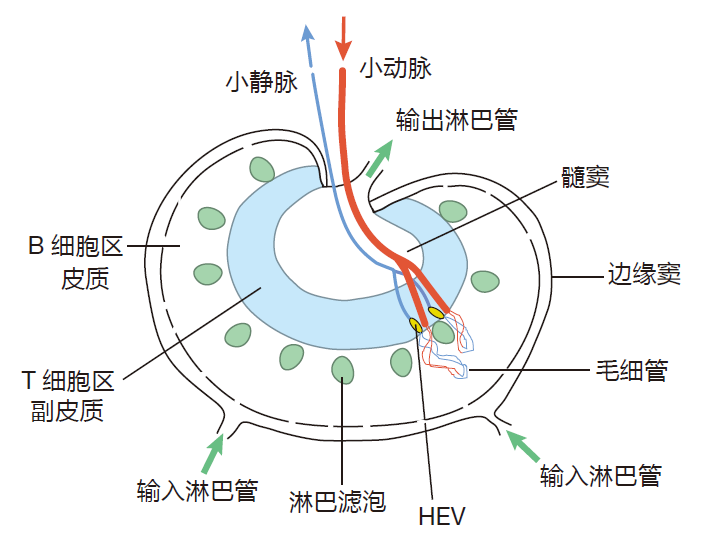
With this diagram in mind, can you see how lymphocytes (B and T cells) enter a lymph node? That's right, they can enter from the blood by pushing their way between the cells of the high endothelial venules. There is also another way lymphocytes can enter the lymph node: with the lymph. After all, lymph nodes are like "dating bars," positioned along the route the lymph takes on its way to be reunited with the blood in the upper torso. And B and T cells actively engage in "bar hopping," being carried from node to node by the lymph. Although lymphocytes have two ways to gain entry to a lymph node, they only exit via the lymph – those high endothelial venules won't let them back into the blood.
Since lymph nodes are places where lymphocytes find their cognate antigen, we also need to discuss how this antigen gets there. When dendritic cells stationed out in the tissues are stimulated by battle signals, they leave the tissues via the lymph, and carry the antigen they have acquired at the battle scene into the secondary lymphoid organs. So this is one way antigen can enter a lymph node: as "cargo" aboard an APC. In addition, antigen which has been opsonized, either by complement or by antibodies, can be carried by the lymph into the node. There the opsonized antigen will be captured by follicular dendritic cells for display to B cells.
When lymph enters a node, it percolates through holes in the marginal sinus (sinus is a fancy word for "cavity"), through the cortex and paracortex, and finally into the medullary sinus – from whence it exits the node via the outgoing lymphatic vessels.
The walls of the marginal sinus are "carpeted" with macrophages that capture and devour pathogens as they enter a lymph node. This substantially reduces the number of invaders that the adaptive immune system will need to deal with, and it helps keep these pathogens from entering the blood stream. This is important, because blood can carry invaders throughout the body, potentially turning a localized infection into a systemic one. So an important function of a lymph node is as a "lymph filter."
The high endothelial venules are located in the paracortex, so B and T cells pass through this region of the node when they arrive from the blood. T cells tend to accumulate in the paracortex, being retained there by adhesion molecules. This accumulation of T cells makes good sense, because dendritic cells also are found in the paracortex – and of course, one object of this game is to get T cells together with these antigen presenting cells. On the other hand, B cells entering a lymph node accumulate in the cortex, the area where lymphoid follicles are located. This localization of B cells works well, because the follicular dendritic cells that display opsonized antigen to B cells are located in this region of the lymph node. So a lymph node is a highly organized place with specific areas for antigen presenting cells, T lymphocytes, B lymphocytes, and macrophages.
Lymph node choreography
The fact that different immune system cells tend to hang out in specific places in a lymph node begs the question: How do they know where to go and when to go there? It turns out that the movements of these cells in this secondary lymphoid organ are carefully choreographed by cytokines called chemokines (short for chemoattractive cytokines). Here's how this works.
Follicular dendritic cells in a lymph node produce a chemokine called CXCL13. Naive B cells which enter the node express receptors for this chemokine, and are attracted to the area of the node where FDCs are displaying opsonized antigen. If a B cell finds its cognate antigen advertised there, it downregulates expression of the receptors for CXCL13, and upregulates expression of another chemokine receptor, CCR7. This receptor detects a chemokine produced by cells in the region of the lymph node where activated Th cells and B cells meet – the border between the B and T cell areas. Consequently, once a B cell has found its antigen, it is attracted by the "smell" of this chemokine to the location in the lymph node where it can receive help from activated Th cells.
Meanwhile, activated Th cells downregulate expression of the chemokine receptors that have been retaining them in the T cell areas. At the same time, they upregulate expression of CXCR5 chemokine receptors, which cause them to be attracted to the border of the follicle – where antigen-activated B cells are waiting for their help. So the movement of immune system cells through a lymph node is orchestrated by the up- and downregulation of chemokine receptors, and the localized production of chemokines that can be detected by these receptors.
Now, of course, human cells don't come equipped with little propellers like some bacteria do, so they can't "swim" in the direction of the source of a chemokine. What human cells do is "crawl." In general terms, the end of the cell that senses the greatest concentration of the chemokine "reaches out" toward the chemokine source, and the other end of the cell is retracted. By repeating this motion, a cell can crawl "up the concentration gradient" toward the source of a cytokine.
At this point, you may be asking, "How do activated Th cells know which B cells to help?" It's a good question with an interesting answer. It turns out that when B cells recognize their cognate antigen displayed by follicular dendritic cells, the B cell's receptors bind tightly to this antigen, and the complex of receptor and cognate antigen is taken inside the B cell. So B cells actually "pluck" antigen from FDC "trees." Once inside the B cell, the antigen is enzymatically digested, loaded onto class II MHC molecules, and presented on the surface of the B cell for Th cells to see. However, to reach full maturity, B cells that have plucked their antigen need co-stimulation. Activated Th cells can provide this co-stimulation because they express high levels of CD40L proteins that can plug into CD40 proteins on the surface of the B cell. But Th cells only provide this stimulation to B cells that are presenting the Th cell's cognate antigen.
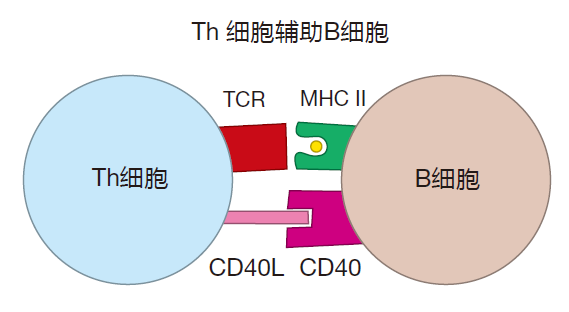
Moreover, Th cells that have been activated by recognizing their cognate antigen also need the assistance of activated B cells in order to mature fully. This assistance involves cell–cell contact during which B7 proteins and proteins called ICOSL on the B cell surface bind to CD28 and ICOS proteins, respectively, on the Th cell surface.
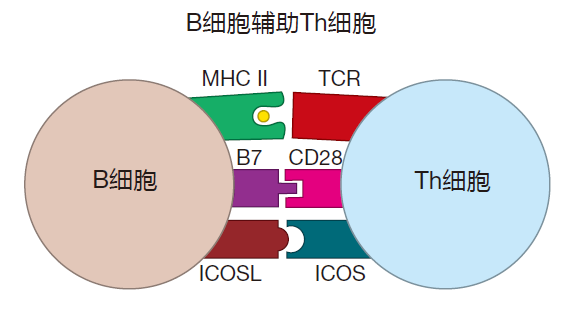
What this means is that at the border of the lymphoid follicle, an activated Th cell and an activated B cell do a "dance" that is critical for their mutual maturation. Th cells provide the CD40L that B cells need. And B cells provide the presented antigen, the B7, and the ICOSL that helper T cells require for their full maturation. Such fully mature Th cells are called follicular helper T (Tfh) cells. These Tfh cells are now "licensed" to rescue fragile germinal center B cells, and to help these B cells switch classes or undergo somatic hypermutation.
The initial encounter between Th and B cells generally lasts about a day, after which some of the B cells proliferate and begin to produce relatively low-affinity IgM antibodies. Although these plasma B cells have not been "upgraded" by class switching or somatic hypermutation, they are important because they provide a relatively fast response to an invasion. Other B cells and their Tfh partners move together into the germinal center, where class switching and somatic hypermutation can take place. Indeed, both class switching and somatic hypermutation usually require the interaction between CD40L proteins on Tfh cells and CD40 proteins on the surface of germinal center B cells.
Somatic hypermutation actually is "driven" by the interaction between germinal center B cells and Tfh cells. B cells with higher affinity receptors are able to "pluck" more antigen from dendritic cells and present more of this antigen via class II MHC molecules to Tfh cells. In return, the B cells receive greater help from these Tfh cells, causing them to proliferate more rapidly when they enter the dark zone of the germinal center. This process enriches the B cell pool in cells with higher affinity BCRs.
It is important to note that during this process of bidirectional stimulation, the part of the protein which the B cell recognizes (the B cell epitope) usually is different from the part of the protein that the Th cell recognizes (the T cell epitope). After all, a B cell's receptors bind directly to a region of the protein which happens to have the right shape to "fit" the B cell's receptors. In contrast, a T cell's receptors bind to a fragment of the protein that has the right amino acid sequence to fit into the groove of an MHC molecule. Consequently, although the B cell epitope and the T cell epitope are "linked" – because they come from the same protein – these epitopes usually are different.
Recirculation through lymph nodes When a T cell enters a lymph node, it frantically checks several hundred dendritic cells, trying to find one that is presenting its cognate antigen. If a T cell is not successful in this search, it leaves the node and continues to circulate through the lymph and blood. If a helper T cell does encounter a dendritic cell presenting its cognate antigen in the paracortex, the Th cell will be activated and will begin to proliferate. This proliferation phase lasts a few days while the T cell is retained in the lymph node by adhesion molecules. During this time, a T cell can have multiple sequential encounters with DCs that are presenting its cognate antigen, increasing the T cell's activation level. The expanded population of T cells then leaves the T cell zone.
Most newly activated Th cells exit the node via the lymph, recirculate through the blood, and enter other lymph nodes via high endothelial venules. This process of recirculation is fast – it generally takes about a day to make the whole circuit – and it is extremely important. Here's why.
There are four major ingredients which must be "mixed" before the adaptive immune system can produce antibodies: APCs to present antigen to Th cells, Th cells with receptors that recognize the presented antigen, opsonized antigen displayed by follicular dendritic cells, and B cells with receptors that recognize the antigen.
Early in an infection there are very few of these ingredients around, and naive B and T cells just circulate through the secondary lymphoid organs at random, checking for a match to their receptors. So the probability is pretty small that the rare Th cell which recognizes a particular antigen will arrive at the very same lymph node that is being visited by the rare B cell with specificity for that same antigen. However, when activated Th cells first proliferate to build up their numbers, and then recirculate to lots of lymph nodes and other secondary lymphoid organs, the Th cells with the right stuff get spread around – so they have a much better chance of encountering those rare B cells which require their help.
B cells which have encountered their cognate antigen displayed on follicular dendritic cells migrate to the border of the lymphoid follicle where they meet activated T cells that have migrated there from the paracortex. It is during this meeting that B cells first receive the co-stimulation they require for activation. Together, the B and Th cells enter the lymphoid follicles, and the B cells proliferate. Many of the newly made B cells then exit the lymphoid follicle via the lymph and become plasma cells. These cells take up residence in the spleen or bone marrow and pump out IgM antibodies. Other activated B cells remain in the lymphoid follicle where they proliferate more and can undergo class switching and additional rounds of somatic hypermutation before they too leave the follicle. In contrast to activated Th cells, activated B cells usually do not recirculate through the lymph and blood and enter other secondary lymphoid organs. Why travel? These B cells have found a secondary lymphoid organ that provides everything they need – antigen displayed on follicular dendritic cells and Tfh cells to help them.
Killer T cells are activated in the paracortex of the lymph node if they find their cognate antigen presented there by dendritic cells. Once activated, CTLs proliferate and recirculate. Some of these CTLs enter other secondary lymphoid organs and begin this cycle again, and others exit the blood at sites of infection to kill pathogen-infected cells.
As everyone knows, lymph nodes that drain sites of infection tend to swell. For example, if you have a viral infection of your upper respiratory tract (e.g., influenza), the cervical nodes in your neck may become swollen. In fact, during a serious infection, lymph nodes can swell to ten times their normal size. This swelling is due in part to the proliferation of lymphocytes within the node.
In addition, cytokines produced by helper T cells in an active lymph node recruit additional macrophages which tend to plug up the medullary sinuses. As a result, fluid is retained in the node, causing further swelling.
The frenzied activity in germinal centers generally is over in about three weeks. By that time, the invader usually has been repulsed, and a lot of the opsonized antigen has been picked from the follicular dendritic trees by B cells. At this point, most B cells will have left the follicles or will have died there, and the areas that once were germinal centers will look much more like primary lymphoid follicles. And your lymph nodes no longer will be swollen.
Interestingly, when surgeons remove a cancer from some organ in the body, they generally inspect the lymph nodes that drain the lymph from that organ. If they find cancer cells in the draining lymph nodes, it is an indication that the cancer has begun to metastasize via the lymphatic system to other parts of the body – the first stop being a nearby lymph node.
In summary, lymph nodes act as "lymph filters" which intercept antigen that arrives from infected tissues either alone or as dendritic cell cargo. These nodes provide a concentrated and organized environment of antigen, APCs, T cells, and B cells in which naive B and T cells can be activated, and experienced B and T cells can be re-stimulated. In a lymph node, naive B and T cells can mature into effector cells that produce antibodies (B cells), provide cytokine help (Th cells), and kill infected cells (CTLs). In short, a lymph node can do it all.
PEYER'S PATCHES
Back in the late seventeenth century, a Swiss anatomist, Johann Peyer, noticed patches of smooth cells embedded in the villi-covered cells that line the small intestine.
We now know that these Peyer's patches are examples of mucosal-associated lymphoid tissues (MALT) which function as secondary lymphoid organs. Peyer's patches begin to develop before birth, and an adult human has about 200 of them. Here is a diagram that shows the basic features of a Peyer's patch.
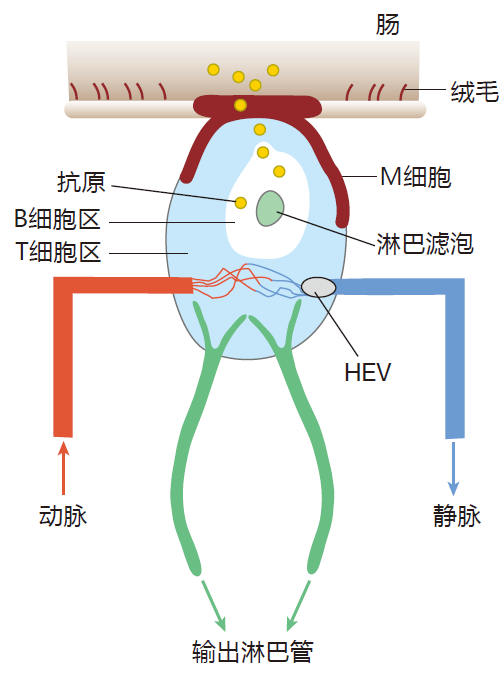
Peyer's patches have high endothelial venules through which lymphocytes can enter from the blood, and, of course, there are outgoing lymphatics that drain lymph away from these tissues. However, unlike lymph nodes, there are no incoming lymphatics that bring lymph into Peyer's patches. So if there are no incoming lymphatics, how does antigen enter this secondary lymphoid organ?
Do you see that smooth cell which crowns the Peyer's patch – the one that doesn't have villi on it? That's called an M cell. These remarkable cells are not coated with mucus, so they are, by design, easily accessible to microorganisms that inhabit the intestine. M cells are "sampling" cells – cells which specialize in transporting antigen from the interior (lumen) of the small intestine into the tissues below. To accomplish this feat, M cells enclose intestinal antigens in vesicles (endosomes). These endosomes are then transported through the M cell, and their contents are spit out into the tissues that surround the small intestine. So, whereas lymph nodes sample antigens from the lymph, Peyer's patches sample antigens from the intestine – and they do it by transporting these antigens through M cells.
Antigen that has been collected by M cells can be carried by the lymph to the lymph nodes that drain the Peyer's patches. Also, if the collected antigen is opsonized by complement or antibodies, it can be captured by follicular dendritic cells in the lymphoid follicles that reside beneath the M cells. In fact, except for its unusual method of acquiring antigen, a Peyer's patch is quite similar to a lymph node, with high endothelial venules to admit B and T cells, and special areas where these cells congregate.
M cells actually are quite selective about the antigens they transport. They don't just take "sips" of whatever is currently within the intestine (how disgusting!).
No, these cells only transport antigens that can bind to molecules on the surface of the M cell. This selectivity makes perfect sense. The whole idea of the M cell and the Peyer's patch is to help initiate an immune response to pathogens that invade via the intestinal tract. And for a pathogen to be troublesome, it has to be able to bind to cells that line the intestines and gain entry into the tissues below. Indeed, most of the stuff we eat will just pass through the small intestine in various stages of digestion without binding to anything. So the minimum requirement for a microbe to be dangerous is that it be able to bind to the surface of an intestinal cell. Consequently, by ignoring all the "non-binders," M cells concentrate the efforts of a Peyer's patch on potential pathogens, and help avoid activating the immune system in response to innocuous food antigens.
THE SPLEEN
The final secondary lymphoid organ on our tour is the spleen. This organ is located between an artery and a vein, and it functions as a blood filter. Each time your heart pumps, about 5% of its output goes through your spleen.
Consequently, it only takes about half an hour for your spleen to screen all the blood in your body for pathogens.
As with Peyer's patches, there are no lymphatics that bring lymph into the spleen. However, in contrast to lymph nodes and Peyer's patches, where entry of B and T cells from the blood occurs only via high endothelial venules, the spleen is like an "open-house party" in which everything in the blood is invited to enter. Here is a schematic diagram of one of the filter units that make up the spleen.
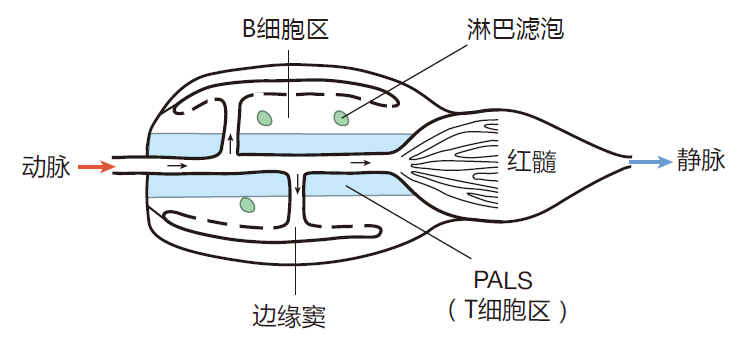
When blood enters from the splenic artery, it is diverted out to the marginal sinuses from which it percolates through the body of the spleen before it is collected into the splenic vein. As they ride along with the blood, naïve B cells and T cells are temporarily retained in different areas – T cells in a region called the periarteriolar lymphocyte sheath (PALS) that surrounds the central arteriole, and B cells in the region between the PALS and the marginal sinuses.
Of course, since the spleen has no lymphatics to transport dendritic cells from the tissues, you might ask, "Where do the antigen presenting cells in the spleen come from?" The answer is that the marginal sinuses, where the blood first enters the spleen, is home to "resident" dendritic cells. These cells take up antigens from invaders in the blood and use them to prepare a class II MHC display.
Resident dendritic cells also can be infected by pathogens in the blood, and can use their class I MHC molecules to display these antigens. Once activated, resident dendritic cells travel to the PALS where T cells have gathered. So although the dendritic cells which present antigens to T cells in the spleen are travelers, their journey is relatively short compared with that of their cousins which travel to lymph nodes from a battle being waged out in the tissues.
Helper T cells that have been activated by APCs in the PALS then move into the lymphoid follicles of the spleen to give help to B cells. And you know the rest of this story!
Some of the most dangerous blood-borne pathogens such as Streptococccus pneumoniae and Haemophilus influenzae surround themselves with a polysaccharide capsule.
Helper T cells can only be activated by protein antigens, so these bacteria, with their carbohydrate cloaks, are "invisible" to helper T cells. Now if B cells in the spleen could not be activated and make antibodies to protect against these dangerous invaders, we'd be in trouble. Fortunately, the spleen is one of the main places in the body where B cells can be activated without the assistance of Th cells. These "helpless" B cells, called marginal zone B cells, are stationed out in the marginal sinuses where they come in contact with blood as it enters the spleen.
And because these marginal zone B cells do not have to wait for T cells to be activated, they can respond quickly before encapsulated bacteria have a chance to multiply to dangerous levels. The importance of this Th-independent B cell activation is underscored by the fact that humans who have lost their spleen (e.g., due to injury) are in danger from serious infections by encapsulated bacteria.
How these marginal zone B cells are activated without T cell help is still a mystery. It likely has to do with the fact that bacterial capsules are composed of many repeating carbohydrate molecules, so there are many epitopes close together to cluster a ton of BCRs. T cell-independent activation also probably depends on B cells using their pattern-recognition receptors and complement receptors to identify these bacteria as being truly dangerous. But nobody knows for sure.
THE LOGIC OF SECONDARY LYMPHOID ORGANS
By now, I'm sure you've caught on to what is going on here. Each secondary lymphoid organ is strategically positioned to intercept invaders that enter the body via different routes. If the skin is punctured and the tissues become infected, an immune response is generated in the lymph nodes that drain those tissues. If you eat contaminated food, an immune response is initiated in the Peyer's patches that line your small intestine. If you are invaded by blood-borne pathogens, your spleen is there to filter them out and to fire up the immune response. And if an invader enters via your respiratory tract, another set of secondary lymphoid organs that includes your tonsils is there to defend you.
Not only are the secondary lymphoid organs strategically positioned, they also provide an environment that is conducive to the mobilization of weapons that are appropriate to the kinds of invaders they are most likely to encounter. Exactly how this works isn't clear yet. However, it is believed that the different cytokines found in the various secondary lymphoid organs determine the local character of the immune response. For example, Peyer's patches specialize in turning out Th cells that secrete a Th2 profile of cytokines as well as B cells that secrete IgA antibodies – weapons that are perfect to defend against intestinal invaders. In contrast, if you are invaded by bacteria from a splinter in your toe, the lymph node behind your knee will produce Th1 cells and their associated cytokines as well as B cells that secrete IgG antibodies – weapons ideal for defending against those bacteria.
Certainly the most important function of the secondary lymphoid organs is to bring lymphocytes and antigen presenting cells together in a way that maximizes the probability that the cells of the adaptive immune system will be activated. Indeed, the secondary lymphoid organs make it possible for the immune system to react efficiently – even when only one in a million T cells is specific for a given antigen. Earlier, I characterized secondary lymphoid organs as dating bars where T cells, B cells, and APCs mingle in an attempt to find their partners. But in fact, it's even better than that. Secondary lymphoid organs actually function more like "dating services." Here's what I mean.
When men and women use a dating service to find a mate, they begin by filling out a questionnaire that records information on their background and their goals.
Then, a computer goes through all these questionnaires and tries to match up people who might be compatible.
In this way, the odds of a man finding a woman who is "right" for him is greatly increased – because they have been preselected. This type of preselection also takes place in the secondary lymphoid organs. These organs are "segregated," with separate areas for naive T cells and B cells. As the billions of Th cells pass through the T cell areas of the secondary lymphoid organs, only a tiny fraction of these cells will be activated – those whose cognate antigens are displayed by the antigen presenting cells that also populate the T cell areas. The Th cells that do not find their antigens leave the secondary lymphoid organs and continue to circulate. Only those lucky Th cells which are activated in the T cell area will proliferate and then travel to a developing germinal center to provide help to B cells.
This makes perfect sense: Allowing useless, non-activated Th cells to enter B cell areas would just clutter things up, and would decrease the chances that Th and B cells which are "right" for each other might get together.
Likewise, many B cells enter the B cell areas of secondary lymphoid organs looking for their cognate antigen displayed by follicular dendritic cells. Most just pass on through without finding the antigen their receptors recognize. Those rare B cells which do find their "mates"
are retained in the secondary lymphoid organs, and are allowed to interact with activated Th cells. Consequently, the "preselection" of lymphocytes in their respective areas of secondary lymphoid organs insures that when Th cells and B cells eventually do meet, they will have the maximum chance of finding their "mates" – just as with a dating service.
LYMPHOCYTE TRAFFICKING
So far, we've talked about the secondary lymphoid organs in which B and T cells meet to do their activation thing, but I haven't said much about how these cells know to go there. Immunologists call this process lymphocyte trafficking. In a human, about 500 billion lymphocytes circulate each day through the various secondary lymphoid organs, but these cells don't just wander around. No, they follow well-defined traffic patterns which maximize their chances of encountering an invader. Importantly, the traffic patterns of virgin and experienced lymphocytes are different. Let's look first at the travels of a virgin T cell.
T cells begin life in the bone marrow and are educated in the thymus (lots more on that subject in Lecture 9). When they emerge from the thymus, virgin T cells express a mixture of cellular adhesion molecules on their surface. These function as "passports" for travel to any of the secondary lymphoid organs. For example, virgin T cells have a molecule called L-selectin on their surface that can bind to its adhesion partner, GlyCAM-1, which is found on the high endothelial venules of lymph nodes.
This is their "lymph node passport." Virgin T cells also express an integrin molecule, α4β7, whose adhesion partner, MadCAM-1, is found on the high endothelial venules of Peyer's patches and the lymph nodes that drain the tissues around the intestines (the mesenteric lymph nodes). So this integrin is their passport to the gut region.
Equipped with an array of different adhesion molecules, inexperienced T cells circulate through all of the secondary lymphoid organs. This makes sense: The genes for a T cell's receptors are assembled by randomly selecting gene segments – so there is no telling where in the body a given naive T cell will encounter its cognate antigen.
In the secondary lymphoid organs, virgin T cells pass through fields of antigen presenting cells in the T cell areas. There these T cells check the billboards on several hundred dendritic cells. If they do not see their cognate antigens advertised, they re-enter the blood either via the lymph or directly (in the case of the spleen), and continue to recirculate. On average, naive T cells make this loop about once a day, spending only about 30 minutes in the blood on each circuit. A naive T cell can continue doing this circulation thing for quite some time, but after about six weeks, if the T cell has not encountered its cognate antigen presented by an MHC molecule, it will die by apoptosis – lonely and unsatisfied. In contrast, those lucky T cells that do find their antigen are activated in the secondary lymphoid organs. These are now "experienced" T cells.
Experienced T cells also carry passports, but they are "restricted passports" because, during activation, expression of certain adhesion molecules on the T cell surface is increased, whereas expression of others is decreased.
This modulation of cellular adhesion molecule expression is not random. There's a plan here. In fact, the cellular adhesion molecules that activated T cells express depend on where these T cells were activated. In this way, T cells are imprinted with a memory of where they came from. For example, DCs in Peyer's patches produce retinoic acid which induces T cells activated there to express high levels of α4β7 (the gut-specific integrin).
As a result, T cells activated in Peyer's patches tend to return to Peyer's patches. Likewise, T cells activated in lymph nodes that drain the skin upregulate expression of receptors that encourage them to return to skin-draining lymph nodes. Thus, when activated T cells recirculate, they usually exit the blood and re-enter the same type of secondary lymphoid organ in which they originally encountered antigen. This restricted traffic pattern is quite logical. After all, there is no use having experienced helper T cells recirculate to the lymph node behind your knee if your intestines have been invaded. Certainly not. You want those experienced helper T cells to get right back to the tissues that underlie your intestines to be re-stimulated and provide help. Equipping activated T cells with restricted passports insures that these cells will go back to where they are most likely to re-encounter their cognate antigens – be it in a Peyer's patch, a lymph node, or a tonsil.
Now, of course, you don't want T cells to just go round and round. You also want them to exit the blood at sites of infection. That way CTLs can kill pathogen-infected cells and Th cells can provide cytokines that amplify the immune response and recruit even more warriors from the blood. To make this happen, experienced T cells also carry "combat passports" (adhesion molecules) which direct them to exit the blood at places where invaders have started an infection. These T cells employ the same "roll, sniff, stop, exit" technique that neutrophils use to leave the blood and enter inflamed tissues. For instance, T cells that gained their experience in the mucosa express an integrin molecule, αEβ7, which has as its adhesion partner an addressin molecule that is expressed on inflamed mucosal blood vessels. As a result, T cells that have the right "training" to deal with mucosal invaders will seek out mucosal tissues which have been infected. In these tis sues, chemokines given off by the soldiers at the front help direct T cells to the battle by binding to the chemokine receptors that were expressed on the surface of the T cells during activation. And when T cells recognize their cognate antigen out in the tissues, they receive "stop" signals which tell them to cease migrating and start defending.
In summary, naive T cells have passports that allow them to visit all the secondary lymphoid organs, but not sites of inflammation. As a consequence, the entire collection of virgin T cells travels through the secondary lymphoid organs, and greatly increases the probability that these T cells will be activated. The reason that virgin T cells don't carry passports to battle sites is that they couldn't do anything there anyway – they must be activated first.
In contrast to virgin T cells, experienced T cells have restricted passports that encourage them to return to the same type of secondary lymphoid organ as the one in which they gained their experience. By recirculating preferentially to these organs, T cells are more likely to be re-stimulated or to find CTLs and B cells that have encountered the same invader and need their help.
And, of course, experienced T cells also have passports that allow them to exit the blood at sites of infection, enabling CTLs to kill infected cells and Th cells to provide appropriate cytokines to direct the battle. This marvelous "postal system," made up of cellular adhesion molecules and chemokines, insures delivery of the right weapons to the sites where they are needed.
B cell trafficking is roughly similar to T cell trafficking.
Like virgin T cells, virgin B cells also have passports that admit them to the complete range of secondary lymphoid organs. However, experienced B cells are not as migratory as experienced T cells. Most just settle down in secondary lymphoid organs or in the bone marrow, produce antibodies, and let these antibodies do the traveling.
WHY MOTHERS KISS THEIR BABIES
Have you ever wondered why mothers kiss their babies?
It's something they all do, you know. Most of the barnyard animals also kiss their babies, although in that case we call it licking. I'm going to tell you why they do it.
The immune system of a newborn human is not very well developed. In fact, production of IgG antibodies doesn't begin until a few months after birth. Fortunately, IgG antibodies from the mother's blood can cross the placenta into the fetus's blood, so a newborn has this "passive immunity" from mother to help tide him over. The newborn can also receive another type of passive immunity: IgA antibodies from mother's milk. During lactation, plasma B cells migrate to a mother's breasts and produce IgA antibodies that are secreted into the milk. This works great, because many of the pathogens a baby encounters enter through his mouth or nose, travel to his intestines, and cause diarrhea. By drinking mother's milk that is rich in IgA antibodies, the baby's digestive tract is filled with antibodies that can intercept these pathogens.
When you think about it, however, a mother has been exposed to many different pathogens during her life, and the antibodies she makes to most of these will not be of any use to the infant. For example, it is likely that the mother has antibodies which recognize the Epstein–Barr virus that causes mononucleosis, but her child probably won't be exposed to this virus until he is a teenager. So wouldn't it be great if a mother could somehow provide antibodies that recognize the particular pathogens that her baby is encountering – and not provide antibodies that the baby has no use for? Well, that's exactly what happens.
When a mother kisses her baby, she "samples" those pathogens that are on the baby's face – the ones the baby is about to ingest. These samples are taken up by the mother's secondary lymphoid organs (e.g., her tonsils), and memory B cells specific for those pathogens are reactivated. These B cells then traffic to the mother's breasts where they produce a ton of antibodies – the very antibodies the baby needs for protection!
REVIEW
In this lecture, we visited three secondary lymphoid organs: a lymph node, a Peyer's patch, and the spleen.
Secondary lymphoid organs are strategically situated to intercept invaders that breach the physical barriers and enter the tissues and the blood. Because of their locations, secondary lymphoid organs play critical roles in immunity by creating an environment in which antigen, antigen presenting cells, and lymphocytes can gather to initiate an immune response. To help make this happen, the secondary lymphoid organs are "compartmentalized" with special areas where T cells or B cells are "preselected" before they are allowed to meet.
B and T cells gain access to a lymph node either from the blood (by passing between specialized high endothelial cells) or via the lymph. Antigen can enter a lymph node with lymph drained from the tissues, so this organ functions as a lymph filter that intercepts invaders. In addition, antigen can be carried to a lymph node as cargo aboard an antigen presenting cell. Within a lymph node, the movements of lymphocytes and dendritic cells are carefully choreographed through the use of cellular adhesion molecules which are up- or downregulated as the cells travel within the node. As a result, helper T cells, which were activated in the T cell areas, move to the boundary of the B cells area to meet with B cells which have recognized their cognate antigen displayed by follicular dendritic cells. There the T and B cells do a "dance," during which the helper T cells become fully "licensed" to help the B cells produce antibodies. These licensed Th cells are called follicular helper T cells (Tfh cells).
Antigen is transported into Peyer's patches through specialized M cells that sample antigen from the intestine. This antigen can interact with B and T cells that have entered the Peyer's patch via high endothelial venules, or it can travel with the lymph to the lymph nodes that drain the Peyer's patch. Thus, a Peyer's patch is a secondary lymphoid organ designed to deal with pathogens which breach the intestinal mucosal barrier.
Finally, we talked about the spleen, a secondary lymphoid organ that is quite different from either a lymph node or a Peyer's patch in that it has no incoming lymphatics and no high endothelial venules. As a result of this "plumbing," antigen and lymphocytes must enter the spleen via the blood. This construction makes the spleen an ideal blood filter that intercepts blood-borne pathogens.
Virgin helper T cells travel though the blood and enter the secondary lymphoid organs. If a Th cell does not encounter its cognate antigen displayed by an APC in the T cell zone, it exits the organ via the lymph or blood (depending on the organ), and visits other secondary lymphoid organs in search of its cognate antigen. On the other hand, if during its visit to a secondary lymphoid organ, a Th cell does find its cognate antigen displayed by class II MHC molecules on a dendritic cell, it becomes activated and proliferates. Most of the progeny then exit the secondary lymphoid organ and travel again through the lymph and the blood. These "experienced" Th cells have adhesion molecules on their surface that encourage them to re-enter the same type of secondary lymphoid organ in which they were activated (e.g., a Peyer's patch or a peripheral lymph node). This restricted recirculation following initial activation and proliferation spreads activated Th cells around to those secondary lymphoid organs in which B cells or CTLs are likely to be waiting for their help. Recirculating Th cells also can exit the blood vessels that run through sites of inflammation. There, Th cells provide cytokines which strengthen the reaction of the innate and adaptive systems to the attack, and which help recruit even more immune system cells from the blood.
Virgin killer T cells also circulate through the blood, lymph, and secondary lymphoid organs. They can be activated if they encounter their cognate antigen displayed by class I MHC molecules on the surface of antigen presenting cells in the T cell zones of the secondary lymphoid organs. Like experienced Th cells, experienced CTLs can proliferate and recirculate to secondary lymphoid organs to be re-stimulated, or they can leave the circulation and enter inflamed tissues to kill cells infected with viruses or other pathogens (e.g., intracellular bacteria).
Virgin B cells also travel to secondary lymphoid organs, looking for their cognate antigens. If they are unsuccessful, they continue circulating through the blood, lymph, and secondary lymphoid organs until they either find their mates or die of neglect. In the lymphoid follicles of the secondary lymphoid organs, a lucky B cell that finds the antigen to which its receptors can bind will migrate to the border of the lymphoid follicle. There, if it receives the required co-stimulation from an activated helper T cell, the B cell will be activated, and will proliferate to produce many more B cells that can recognize the same antigen. All this activity converts a primary lymphoid follicle, which is just a loose collection of follicular dendritic cells and B cells, into a germinal center in which B cells proliferate and mature. In a germinal center, B cells may class switch to produce IgA, IgG, or IgE antibodies, and they may undergo somatic hypermutation to increase the average affinity of their receptors for antigen.
These two "upgrades" usually require the ligation of CD40 on the maturing B cells by CD40L proteins on Tfh cells.
Most of these B cells then become plasma cells and travel to the spleen or bone marrow, where they produce antibodies. Others remain in the germinal center and undergo further rounds of proliferation and selection.


