第8讲 Restraining the Immune System
HEADS UP!
In some situations, a vigorous immune response is not desirable, and the immune system must be restrained so that it does not become overexuberant. Also, after the immune system has vanquished an intruder, production of the weapons used to defend against that invader must be stopped, and most of those weapons must be destroyed.
INTRODUCTION
The immune system evolved to provide a rapid and overwhelming response to invading pathogens. After all, most attacks by viruses or bacteria result in acute infections which either are quickly dealt with by the immune system (in a matter of days or weeks) or overwhelm the immune system and kill you. Built into this system are positive feedback loops in which various immune system players work together to get each other fi red up. However, once an invasion has been repulsed, these feedback loops must be broken, and the system must be turned off.
In addition, there are times when a vigorous response to an invasion simply is not appropriate, and in those situations, the immune system must be restrained in order to prevent irreparable damage to our bodies.
Until recently, immunologists spent most of their effort trying to understand how the immune system gets turned on, and great progress has been made in that area. Now, however, many immunologists are focusing on the equally important question of how the system is restrained.
ATTENUATING THE IMMUNE RESPONSE
We generally think of helper T cells as being important in activating the immune system. However, another type of CD4 + T cell has been discovered which actually can dampen the immune response. This is called the inducible regulatory T cell (iTreg). These T cells are termed "inducible" because, just as naive helper T cells can be encouraged to become Th1, Th2, or Th17 cells, naive Th cells activated in an environment that is rich in TGFβ can be "induced" to become iTregs. Inducible regulatory T cells are called "regulatory" because, instead of secreting cytokines such as TNF and IFN-γ, which activate the immune system, iTregs produce cytokines such as IL-10 and TGFβ that help restrain the system.
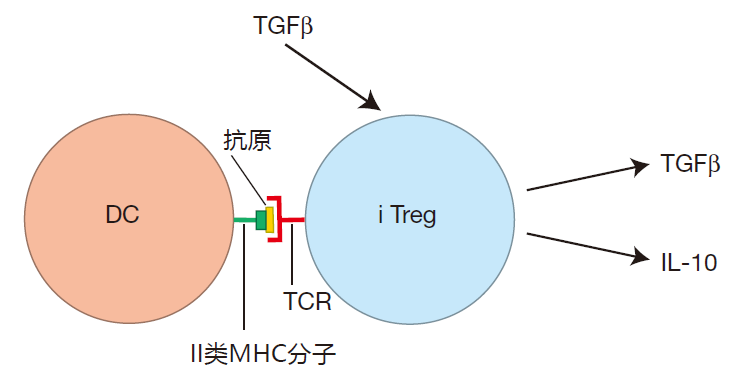
When IL-10 binds to its receptors on antigen presenting cells, it reduces the expression of the APC's pattern recognition receptors, making it more difficult for these APCs to be activated. Also, IL-10 binding to APCs reduces the levels of the B7 co-stimulatory molecules that are expressed on the APC's surface. And this makes it harder for the APC to activate T cells. In addition, TGFβ produced by iTregs reduces the proliferation rate of T cells, and also makes killer T cells less vicious killers.
The net result is that the cytokines produced by iTregs can attenuate the immune response and prevent excessive immune activation.
One area of our body where preventing immune over-exuberance is extremely important is in the tissues that underlie the intestines. Our intestines are home to trillions of harmless bacteria, and inducible regulatory T cells play a major role in keeping the warriors that guard the intestines from overreacting to these bacteria. Intestinal immunity is the subject of Lecture 11.
It also is believed that iTregs are important in protecting us against allergies caused by an overreaction of the immune system to common environmental antigens. In this case, iTregs are thought to act, at least in part, by inhibiting mast cell degranulation – an event which is central to the allergic reaction. We will talk more about allergies in Lecture 13.
DEACTIVATING THE SYSTEM
Even in situations in which it is appropriate for the immune system to react strongly against invaders, immune warriors still must be restrained once the battle has been won.
During an invasion, as the immune system gains the upper hand and the intruders are destroyed, there will be less and less "invading antigen" present. Consequently, fewer innate system cells will be activated, and fewer dendritic cells will mature and travel to secondary lymphoid organs with their cargo of battle antigens. So as foreign antigen is eliminated, the level of activation of both the innate and the adaptive system decreases. This is the first step in turning off the immune system.
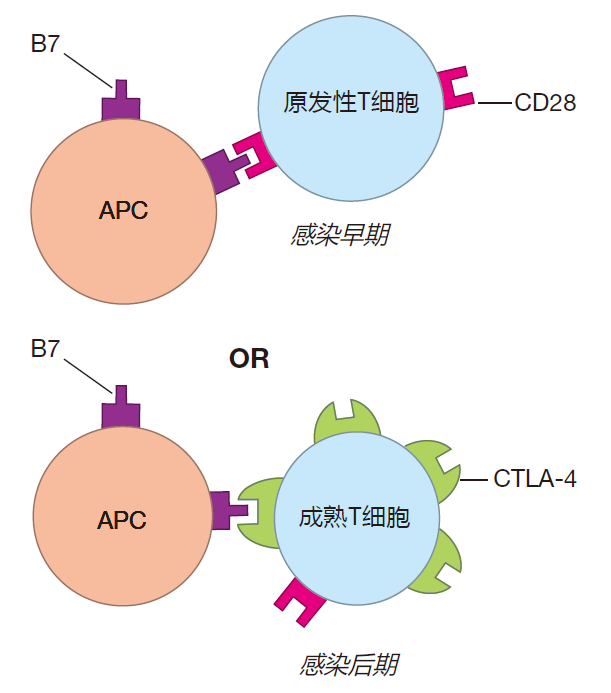
Although the removal of foreign antigen is very important, other mechanisms also help decrease the level of activation as the battle winds down. In Lecture 4 we discussed the B7 co-stimulator protein. Activation of a T cell requires that, in addition to ligation of its T cell receptors, B7 proteins on the surface of the APC must plug into CD28 molecules on the surface of the T cell. This co-stimulatory signal greatly increases the efficiency of T cell activation. However, in addition to engaging stimulatory CD28 molecules on T cells, B7 proteins on APCs also can plug into other receptor proteins on T cells called CTLA-4. Although most human T cells continuously display CD28 on their surface, the bulk of a naive T cell's CTLA-4 is stored inside the cell. Then, beginning about two days after a virgin T cell is first activated, more and more CTLA-4 is moved from these intracellular reservoirs to the cell surface. Importantly, B7 on antigen presenting cells binds to CTLA-4 with an affinity thousands of times higher than its affinity for CD28. Consequently, as time goes on, the CTLA-4 molecules out-compete CD28 for B7 binding. As a result, early in an infection, B7 binds to CD28 and acts as a co-stimulator. Then, after the battle has been raging for a while, the limited number of B7 proteins on an APC bind mainly to CTLA-4, not CD28.
This makes it harder for these T cells to be reactivated, and helps shut down the adaptive immune response.
Another molecule (with a great name!), programmed death 1 (PD-1), also can help deactivate T cells. Like CTLA-4, expression of PD-1 on the surface of T cells increases after activation. The ligand for PD-1, PD-L1, appears on the surface of many different cell types in tissues which are under attack (inflamed tissues). When the PD-L1 protein on inflamed tissues binds to PD-1 on T cells that have been at work for a while, the T cells become "lethargic" – so that they don't function well. This helps minimize the "collateral damage" that might occur if T cells were not restrained once an infection has been dealt with.
In summary, late in an infection, CTLA-4 "soaks up" B7 co-stimulatory proteins on APCs and makes reactivation of T cells less efficient. Ligation of PD-1 inhibits the function of previously activated T cells. Together, CTLA-4 and PD-1 function as checkpoint proteins which help "decommission" T cells as the battle winds down.
These checkpoint proteins probably should have been called "negative immune regulator" proteins – because that's really what they are.
LIFE IS SHORT
As a consequence of the removal of foreign antigen and the subsequent cessation of activation, the immune system will stop producing those weapons which can defend against a banished invader. Nevertheless, many of the weapons made during the struggle will remain at the battle site, and these stockpiles of obsolete weapons must somehow be eliminated. Fortunately, this problem is partly solved by making many of these weapons short-lived.
During a major invasion, huge numbers of neutrophils are recruited from the blood, but these cells are programmed to die after a few days. Likewise, natural killer cells have a half-life of only about a week. Consequently, once recruitment ceases, the stockpiles of neutrophils and NK cells are quickly depleted. Moreover, because natural killer cells supply IFN-γ to help keep macrophages fired up, when NK cells die off, macrophages tend to go back to a resting state.
Dendritic cells, once they reach a lymph node, only live about a week, and plasma B cells die after about five days of hard labor. Consequently, as the activation of Th and B cells wanes, the number of plasma B cells specific for an invader declines. In addition, the antibodies which plasma cells produce have short lifetimes, with the longest lived (the IgG class) having a half-life of only about three weeks.
As a result, once plasma B cells stop being produced, the number of invader-specific antibodies drops rapidly.
EXHAUSTION
Although many immune system weapons are short-lived, T cells are an important exception to this "rule." In contrast to cells such as neutrophils, which are programmed to self-destruct after a short time on the job, T cells are designed to live a long time. The reason for this is that naive T cells must circulate again and again through the secondary lymphoid organs, looking for their particular antigen on display. Consequently, it would be extremely wasteful if T cells were short-lived. On the other hand, once T cells have been activated, have proliferated in response to an attack, and have defeated the invader, the longevity of T cells could be a major problem. Indeed, at the height of some viral infections, more than 10% of all our T cells recognize that particular virus. If most of these cells were not eliminated, our bodies would soon fill up with obsolete T cells that could only defend us against invaders from
the past. Fortunately, this problem is solved by activation-induced cell death (AICD) – a way of eliminating obsolete T cells after they have been re-stimulated many times in the course of a battle. Here's how this works.
CTLs have proteins called Fas ligand that are prominently displayed on their surface, and one way they kill is by plugging this protein into its binding partner, Fas, which is present on the surface of target cells.
When these proteins connect, the target is triggered to commit suicide by apoptosis. Virgin T cells are "wired" so that they are insensitive to ligation of their own Fas proteins. However, when T cells are activated and then reactivated many times during an attack, their internal wiring changes. During this process, they become increasingly sensitive to ligation of their Fas proteins by their own Fas ligand proteins or by FasL on other T cells. This feature makes these "exhausted" T cells targets for Fas-mediated killing – either by suicide or homicide. By this mechanism, activation-induced cell death eliminates T cells which have been repeatedly activated, and makes room for new T cells that can protect us from the next microbes which might try to do us in. In fact, once an invader has been vanquished, more than 90% of the T cells which responded to the attack usually die off.
REVIEW
Inducible regulatory T cells (iTregs) are helper T cells which secrete cytokines designed to keep the immune system "calm" when we are not threatened by dangerous invaders.
And after a real threat has been dealt with, it is important to turn the immune system off and dispose of obsolete weapons. Continued activation of the system depends on the presence of foreign antigen, so as invaders are destroyed, the activation level of the system decreases. Moreover, T cells that have been repeatedly reactivated express checkpoint proteins on their surface. These "negative regulators" make it more difficult to reactivate T cells (CTLA-4)
or make T cells function less well (PD-1). In addition, the short lifetimes of many immune warriors help reduce the stockpiles of weapons that are no longer needed, and T cells that are "exhausted" from their efforts are eliminated by activation-induced cell death. All of these mechanisms combine to "reset" the system after each infection, so that it will be ready to deal with the next attack.


