第9讲 Self Tolerance和MHC限制性
HEADS UP!
T cells must be "restricted" to recognize self MHC molecules, so that the attention of these cells will be focused on MHC–peptide complexes, not on unpresented antigen. In addition, B cells and T cells must be screened to eliminate those which might attack our own bodies. The safeguards that protect against autoimmunity are multilayered, with each layer designed to catch potentially self-reactive cells that "slip through the cracks" in the layers above. Natural killer cells also are tested to be sure they do not cause autoimmune disease.
INTRODUCTION
The subject of this lecture is one of the most exciting in all of immunology. Part of that excitement arises because, although a huge amount of research has been done on tolerance of self and MHC restriction, there are still many unanswered questions. But what really makes this topic interesting is that it is so important. B cells and T cells must learn not to recognize our own antigens as dangerous. Otherwise, we would all die of autoimmune disease.
THE THYMUS
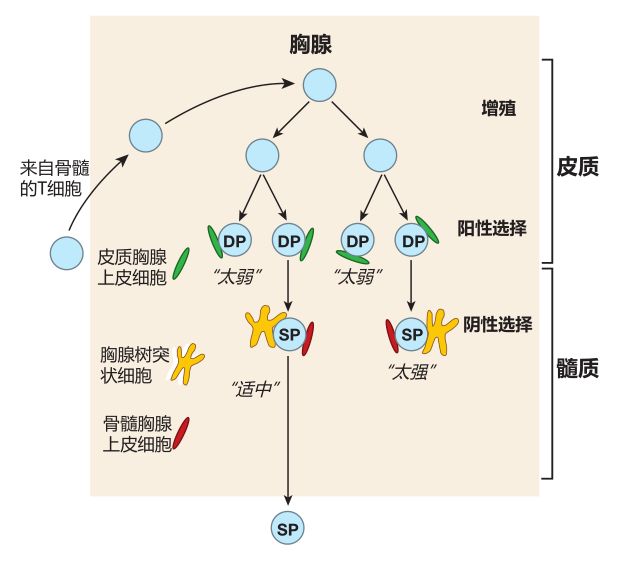
T cells first learn tolerance of self in the thymus, a small organ located just below the neck. This process usually is called central tolerance induction. Like the spleen, the thymus has no incoming lymphatics, so cells enter the thymus from the blood. However, in contrast to the spleen, which welcomes anything that is in the blood, entry of cells into the thymus is quite restricted. It is believed that immature T cells from the bone marrow enter the thymus in waves, somewhere in the middle of this organ. However, exactly how this happens is not understood, because the high endothelial cells that allow lymphocytes to exit the blood into secondary lymphoid organs are missing from the thymus.
What is known is that the T cells enter the thymus from the bone marrow "in the nude": They don't express CD4, CD8, or a TCR. After entry, these cells migrate to the outer region of the thymus (the cortex ) and begin to proliferate.
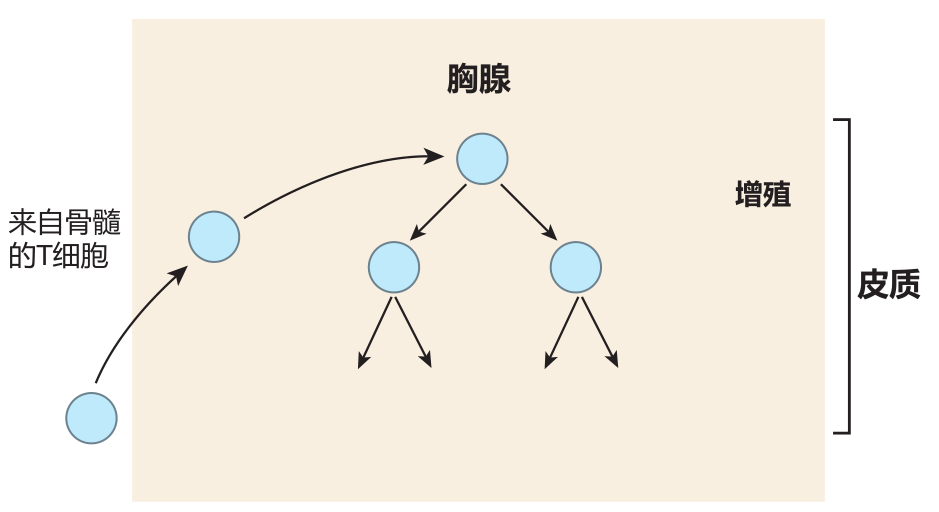
About this time, some of the T cells start to rearrange the gene segments that encode the α and β chains of the TCR. If these rearrangements are successful, a T cell begins to express low levels of the T cell receptor (including the CD3 protein complex) as well as the CD4 and CD8 co-receptors. As a result, the formerly nude T cells soon are "dressed" with CD4, CD8, and TCR molecules on their surface. Because these T cells express both CD4 and the CD8, they are called double-positive (DP) cells.
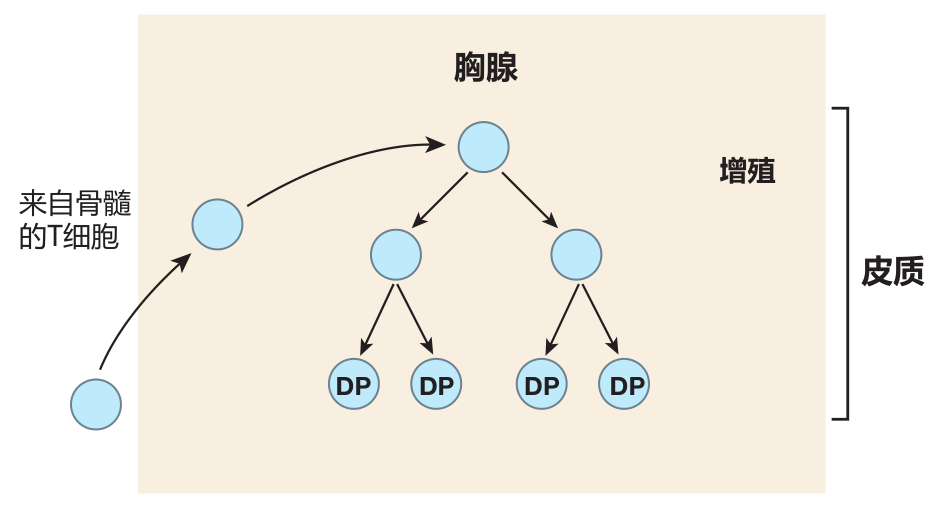
During this "reverse striptease," another important change takes place. When the T cell was naked, it was resistant to death by apoptosis because it expressed little or no Fas antigen (which can trigger cell death when ligated), and because it expressed high levels of Bcl-2 (a cellular protein that protects against apoptosis). In contrast, a "fully dressed" T cell of the thymic cortex expresses high levels of Fas on its surface and produces very little Bcl-2. Consequently, it is exquisitely sensitive to signals that can trigger death by apoptosis. It is in this highly vulnerable condition that a T cell is tested for MHC restriction and tolerance of self. If it fails either test, it will die a horrible death!
MHC RESTRICTION
The process of testing T cells for MHC restriction is usually referred to as positive selection. The "examiners" here are epithelial cells in the cortical region of the thymus, and the question a cortical thymic epithelial cell (cTEC) asks of a T cell is: Do you have receptors that recognize one of the self MHC molecules which I am expressing on my surface? The correct answer is, "Yes, I do!" for if its TCRs do not recognize any of these self MHC molecules, the T cell dies.
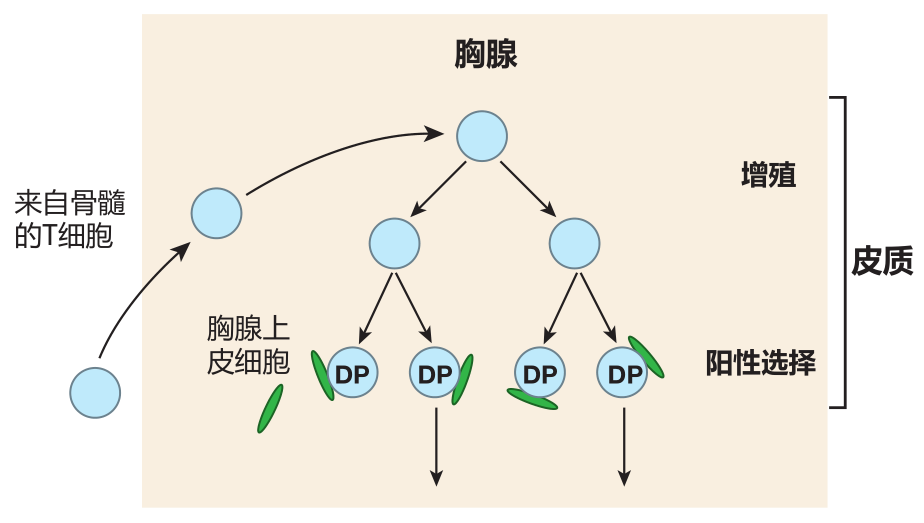
When I say "self" MHC, I simply mean those MHC molecules which are expressed by the person (or mouse) who "owns" this thymus. Yes, that does seem like a no-brainer – that my T cells would be tested in my thymus on my MHC molecules – but immunologists like to emphasize this point by saying "self MHC."
The MHC molecules on the surface of the cortical thymic epithelial cells actually are loaded with peptides, so what a TCR really recognizes is the combination of a self MHC molecule and its associated peptide. The peptides presented by the cTEC's class I MHC molecules represent a sampling of the proteins that are being made inside the cell. This is normal class I presentation. Cortical thymic epithelial cells use their class II MHC molecules to present fragments of proteins which they have taken up from the environment within the thymus. This is normal class II MHC presentation. However, immunologists have recently discovered that cortical thymic epithelial cells also employ their class II MHC molecules to present many peptides which don't come from outside these cells.
This is what you might call "abnormal" class II MHC presentation. Here's how this works.
Cells have evolved several mechanisms to help them deal with times of famine – situations when the raw materials required for the synthesis of cellular components are limiting. One such survival tool is a process called autophagy (literally "self eating"). When cells are starving, they can enclose portions of their cytoplasm in membranes, which then fuse with lysosomes. The cytoplasmic components (e.g., proteins) are then disassembled by lysosomal enzymes so that they can be reused. Remarkably, cortical thymic epithelial cells also can employ autophagy to capture their own intracellular proteins, digest them into short peptides, and display them on their surface using class II MHC molecules. By using autophagy to prepare this abnormal display, cortical thymic epithelial cells greatly increase the universe of self peptides they can present to T cells in the thymus. Presumably, this makes it more likely that a T cell will see a combination of a class II MHC molecule and a peptide to which it can bind – and therefore be positively selected for survival.
THE LOGIC OF MHC RESTRICTION
Let's pause for a moment between exams to ask an important question: Why do T cells need to be tested to be sure that they can recognize peptides presented by self MHC molecules? After all, most humans complete their lifetimes without ever seeing "foreign" MHC molecules (e.g., on a transplanted organ), so MHC restriction can't be about discriminating between your MHC molecules and mine. No, MHC restriction has nothing to do with foreign versus self – it's all about focus. As we discussed in Lecture 4, we want the system to be set up so that T cells focus on antigens that are presented by MHC molecules. Like a B cell's receptors, a T cell's receptors are made by mixing and matching gene segments, so they are incredibly diverse. As a result, it is certain that in the collection of TCRs expressed on T cells, there will be many which recognize unpresented antigens, just as a B cell's receptors do. These T cells must be eliminated.
Otherwise the wonderful system of antigen presentation by MHC molecules won't work. So the reason positive selection (MHC restriction) is so important is that it sets up a system in which all mature T cells will have TCRs that recognize antigen presented by self MHC molecules.
THYMIC TESTING FOR TOLERANCE OF SELF
During or slightly after positive selection takes place in the cortex of the thymus, T cells stop displaying either one or the other of the co-receptor molecules, CD4 or CD8. As you'd predict, these cells are then called single-positive (SP) cells. The exact mechanism by which a T cell "chooses" between displaying CD4 or CD8 co-receptors is still being explored. However, the emerging picture is that the choice of co-receptor depends on whether a particular T cell recognizes its cognate antigen displayed by either the class I or class II MHC molecules on a cortical thymic epithelial cell. For example, if a T cell's receptors recognize an antigen displayed by class I MHC molecules, CD8 co-receptors on the T cell surface will "join the party" and clip onto the MHC molecule.
When this happens, the expression of CD4 molecules on that T cell is downregulated. And similarly, a T cell whose receptors recognize a peptide displayed by class II molecules will become a CD4 T cell, and expression of CD8 co- receptors on that cell will be turned off. This strategy works because CD8 co-receptors only bind to class I MHC molecules, and CD4 co-receptors only bind to class II MHC molecules.
Those lucky T cells whose TCRs recognize self MHC plus peptide begin to express the CCR7 chemokine receptor on their surface, and proceed from the thymic cortex to the central region of the thymus called the medulla – where ligands for CCR7 are plentiful. It is in the thymic medulla that the second test is administered: the test for tolerance of self. This exam is frequently referred to as negative selection.
The exam question asked of T cells during negative selection is: Do you recognize any of the self peptides displayed by the MHC molecules on my surface? The correct answer is, "No way!" because T cells with receptors that do recognize the combination of MHC molecules and self peptides are deleted. This second test, which eliminates T cells that could react against our own antigens, is crucial.
Indeed, if such self-reactive T cells were not deleted, autoimmune disease could result. For example, Th cells that recognize self antigens could help B cells make antibodies that would tag our own molecules (e.g., the insulin proteins in our blood) for destruction – or CTLs could be produced that would attack our own cells.
Medullary thymic epithelial cells
There are two types of cells that screen for tolerance of self antigens, and both cell types are different from the cortical thymic epithelial cells that tested T cells for MHC restriction (positive selection). One cell type involved in testing T cells for tolerance of self is the medullary thymic epithelial cell (mTEC). These cells are cousins of the cortical thymic epithelial cells that test for MHC restriction, and they have two properties which make them especially suited as "tolerance testers." First, like cortical thymic epithelial cells, mTECs use autophagy to digest their own "innards" and process these proteins for presentation by class II MHC molecules. This rule-breaking presentation, in which proteins made within the cell are displayed by class II MHC molecules, provides a diverse source of self antigens that can be used to eliminate most self-reactive helper T cells during negative selection.
However, there still is a problem. In addition to the "shared" proteins which all cells produce, there are many proteins (estimates suggest several thousand) that are "tissue-specific." These tissue-specific proteins are the ones which give each organ or tissue type its identity. For example, there are proteins produced by the cells that make up your heart which are unique to that organ. Also, there are proteins made by kidney cells that are kidney-specific. So for tolerance testing in the thymus to be complete, tissue-specific proteins would need to be included in the "material" on which student T cells are examined. Otherwise, when killer T cells leave the thymus, some of them would surely encounter tissue-specific proteins to which they were not tolerant – and set about destroying your liver, your heart, or your kidneys. Not good.
Fortunately, medullary thymic epithelial cells produce a transcription factor called AIRE that drives expression of many tissue-specific antigens. Consequently, medullary thymic epithelial cells express, in addition to the usual shared proteins, more than a thousand tissue-specific proteins. This certainly helps solve the problem of eliminating T cells with receptors that might recognize tissue-specific proteins. However, there is still uncertainty surrounding the issue of tolerance to tissue-specific antigens. For example, it is not known whether mTECs express all of the tissue-specific proteins found in the body or just most of them.
Thymic dendritic cells
There is a second cell type which has been implicated in testing for tolerance of self antigens in the thymus: the thymic dendritic cell (TDC). Although thymic DCs have a characteristic starfish-like shape, they are different from the "migratory" dendritic cells we have discussed previously. The medullary TDCs are "residents" of the thymic medulla and develop there from bone-marrow-derived precursors. What is interesting about TDCs is that in addition to acquiring antigens in the usual way from the thymic environment, some of the antigens they present are "given" to them by mTECs. Indeed, it appears that MHC–peptide complexes from mTECs are somehow "handed off" to thymic dendritic cells for them to use to test CD4 + and CD8 + cells for tolerance of self. How this handoff is accomplished, and why it is important remains a mystery. Clearly, there is still a lot still to be discovered about negative selection in the thymus!
GRADUATION
The final result of all this testing in the thymus is a collection of T cells that has receptors which do recognize self MHC–peptide complexes presented by cortical thymic epithelial cells, but which do not recognize self antigens presented by MHC molecules on thymic dendritic cells or medullary thymic epithelial cells.
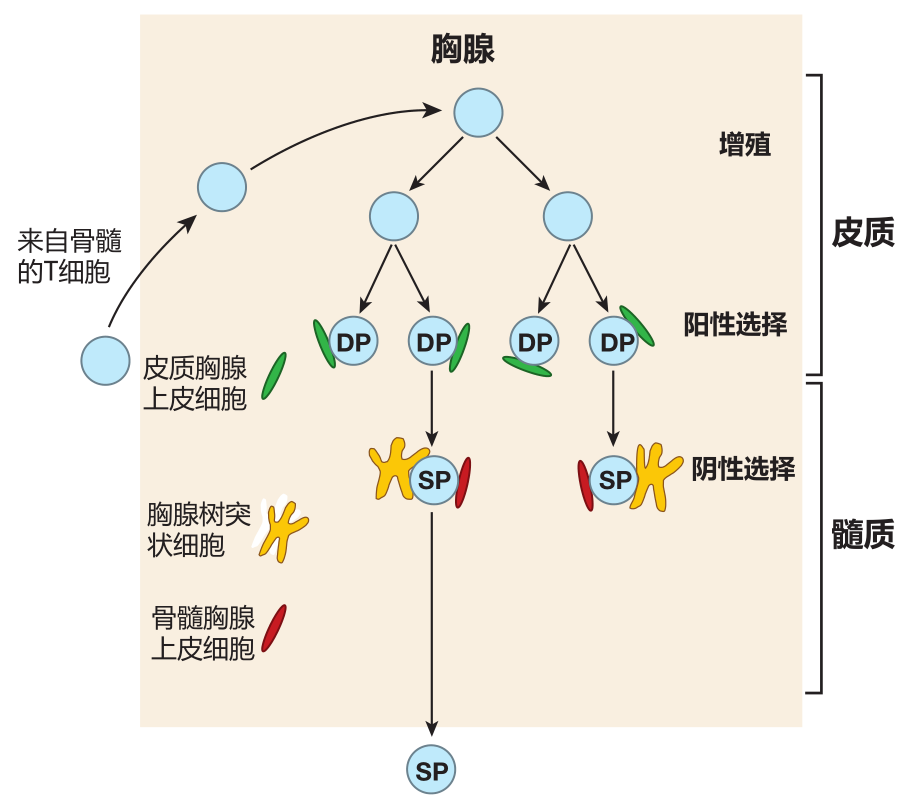
The "thymic graduates" that pass these tests express high levels (i.e., many molecules) of the T cell receptor on their surface, plus either the CD4 or CD8 co-receptor, but not both. Each day in the thymus of a young person, about 60 million double-positive cells are tested, but only about 2 million single-positive cells exit the thymus.
The rest die by apoptosis, and are quickly eaten by macrophages in the thymus. Most students are not too thrilled about exams that last more than an hour, so I thought you might like to know that these tests take about two weeks!
We're talking major exams here, where the life of each T cell hangs in the balance. Interestingly, immunologists still aren't certain how these graduates leave the thymus, but it is thought that they exit near the corticomedullary junction via the blood.
THE RIDDLE OF MHC RESTRICTION AND TOLERANCE INDUCTION
Now, if you've been paying close attention, you may be wondering how any T cells could possibly pass both exams. After all, to pass the test for MHC restriction, their TCRs must recognize MHC plus self peptide. Yet to pass the tolerance exam, their TCRs must not be able to recognize MHC plus self peptide. Doesn't it seem that the two exams would cancel each other out, allowing no T cells to pass? It certainly does, and this is the essence of the riddle of self tolerance: How can ligation of a T cell receptor possibly result in both positive selection (MHC restriction) and negative selection (tolerance induction)? In fact, it is even more complicated than that, because once a T cell has passed both tests in the thymus, its TCRs must be able to signal activation when they encounter invader-derived peptides presented by self MHC molecules.
The "Goldilocks" hypothesis
It is known that the events leading to MHC restriction and tolerance induction are similar to those involved in the activation of T cells: cell–cell adhesion, TCR clustering, and co-stimulation. However, the question that vexes immunologists is: How does the same TCR, when it engages MHC–peptide complexes, signal three very different outcomes – positive selection, negative selection, or activation? Unfortunately, I can't answer this question (otherwise I'd be on my way to Sweden to pick up my Nobel Prize), but immunologists favor the "affinity model" or what could be called the Goldilocks hypothesis. This hypothesis states that to survive both positive and negative selection in the thymus, T cells must have receptors that are "just right." Indeed, it is hypothesized that in the thymus, positive selection (survival) of T cells results from a relatively weak interaction between TCRs and MHC–self peptide displayed on cortical thymic epithelial cells – an interaction that is strong enough to insure that the TCRs are focused on presented antigen.
Then, the interaction between TCRs and MHC–self peptide expressed on medullary thymic epithelial cells or thymic dendritic cells must not be too strong or cell death (negative selection) will result. And finally, after T cells leave the thymus, the interaction between their TCRs and MHC–peptide displayed by professional antigen presenting cells must be strong enough to trigger activation.
Of course, since the TCR is the same in all three situations, the question is what makes the effect of these three interactions of MHC–peptide with a T cell receptor so different: life, death, or activation? One key element appears to be the properties of the cell that "sends" the signals. In the case of MHC restriction, this is a cortical thymic epithelial cell. For tolerance induction, the cell is a bone marrow-derived dendritic cell or a medullary thymic epithelial cell. And for activation, the sender is a specialized antigen presenting cell. These sender cells are very different. For example, the proteasomes of cortical thymic epithelial cells – the machines that chop up proteins to make peptides for class I display – are subtly different from the proteasomes of the cells that are responsible for negative selection. This could affect which self peptides are presented on class I MHC molecules by these examiner cells. Moreover, some of the enzymes that cortical thymic epithelial cells use to prepare peptides for presentation on class II MHC molecules are different from the corresponding enzymes employed by the examining cells in the thymic medulla.
It is also likely that the various sender cells differ in the cellular adhesion molecules they express and in the number or type of MHC–peptide complexes they display on their surface. Such differences could dramatically influence the strength of the signal that is sent through the T cell receptor. In addition, the different types of sender cells are likely to express different mixtures of co-stimulatory molecules – and co-stimulatory signals could change the meaning of the signal that results from TCR–MHC–peptide engagements.
Not only are the cells that send the signals different, the "receiver" (the T cell) also may change between exams. It is known that the number of TCRs on the surface of the T cell increases as the cell is tested, and it is also possible that the "wiring" within the T cell changes as the T cell matures. These differences in TCR density and signal processing could influence the interpretation of signals generated by the various types of sender cells.
Although many of the pieces of the MHC restriction/tolerance induction puzzle have been found, immunologists still have not been able to assemble them into a completely consistent picture. More work is required.
TOLERANCE BY IGNORANCE
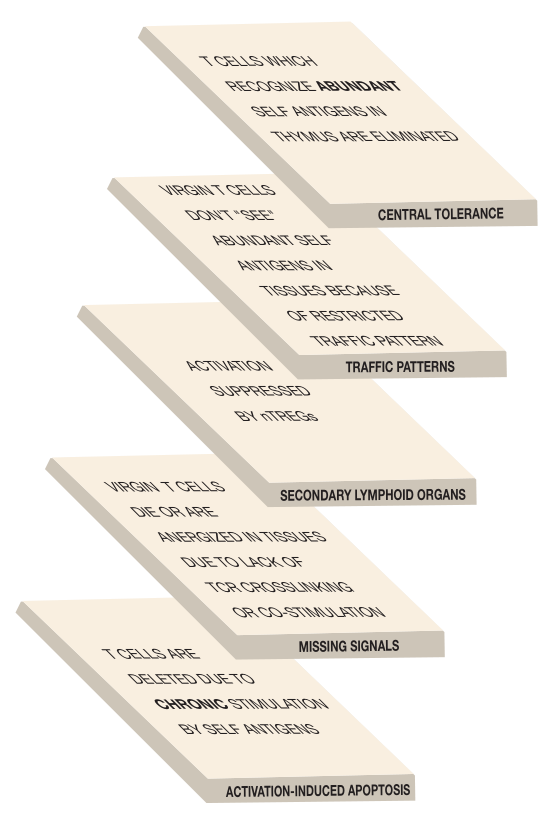
Thankfully, most T cells with receptors which could recognize our own proteins are eliminated in the thymus. However, central tolerance induction in the thymus is not foolproof. If it were, every single T cell would have to be tested on every possible self antigen – and that's a lot to ask.
The probability is great that T cells with receptors which have a high affinity for those self antigens that are abundant in the thymus will be deleted there. However, T cells whose receptors have a low affinity for self antigens, or which recognize self antigens that are rare in the thymus, are less likely to be negatively selected. They may just "slip through the cracks" of central tolerance induction. Fortunately, the system has been set up to deal with this possibility. Virgin T cells circulate through the secondary lymphoid organs, but are not allowed out into the tissues.
This traffic pattern takes these virgins to the areas of the body where they are most likely to encounter APCs and be activated. However, the travel restriction that keeps virgin T cells out of the tissues also is important in maintaining self tolerance. The reason is that, as a rule, those self antigens which are abundant in the secondary lymphoid organs, where virgin lymphocytes are activated, also are abundant in the thymus, where T cells are tolerized. Therefore, as a result of the traffic pattern followed by virgin T cells, most T cells that could be activated by an abundant self antigen in the secondary lymphoid organs already will have been eliminated by seeing that same, abundant self antigen in the thymus.
Conversely, T cells whose receptors recognize self antigens that are relatively rare in the thymus may escape deletion there. However, these same antigens usually exist at such low concentrations in the secondary lymphoid organs that they do not activate potentially self-re-active T cells. Thus, although rare self antigens are present in the secondary lymphoid organs, and although T cells do have receptors which can recognize them, these T cells usually remain functionally "ignorant" of their presence – because the self antigens are too rare to trigger activation. So lymphocyte traffic patterns play a key role not only in insuring the efficient activation of the adaptive immune system, but also in preserving tolerance of self antigens.
TOLERANCE INDUCTION IN SECONDARY LYMPHOID ORGANS
Although the restricted traffic pattern of naive T cells usually protects them from exposure to self antigens which might activate them, this barrier to activation is not absolute. Occasionally, self antigens that are too rare in the thymus to cause deletion of potentially autoreactive T cells are released into the blood and lymphatic systems (e.g., as the result of an injury which causes tissue damage) in concentrations sufficient to activate previously ignorant T cells. But again, the immune system has a way to deal with this potential problem.
Until recently, it was thought that the thymus' only role in preventing autoimmunity was the elimination of potentially self-reactive T cells. However, it now is clear that there is an additional thymic function which helps protect us from autoimmune disease – the generation of natural regulatory T cells (nTregs). In the thymus, a subset of CD4 + T cells is selected to become natural regulatory T cells. This selection takes place in the thymic medulla and requires the action of mTECs and TDCs.
The current thinking is that CD4 + T cells whose receptors have a strong affinity for self antigens presented by these cells are eliminated. T cells whose receptors have a weak affinity for self antigens presented by mTECs or TDCs are selected to survive as helper T cells. And CD4 + T cells with an "intermediate" affinity for self antigens are "induced" to become natural regulatory T cells. However, exactly what "intermediate" means in this context and the details of how these T cells are "induced" are not known.
One thing that is known about the selection of CD4 + T cells to become nTregs is that these cells are somehow induced to express a gene called Foxp3, which is instrumental in conferring upon nTreg cells their regulatory properties. After they are generated in the thymus, natural Tregs receive passports (adhesion molecules) which allow them to enter lymph nodes and other secondary lymphoid organs. Indeed, about 5% of all the CD4 + T cells in circulation are regulatory T cells. If, in a secondary lymphoid organ, a natural Treg encounters its cognate self antigen presented by an antigen presenting cell, it can be activated. Once activated, nTreg cells are able to suppress the activation of potentially self-reactive T cells. Exactly how they accomplish this is still unclear. One likely mechanism is that when a Treg cell recognizes its cognate antigen displayed by an antigen presenting cell, it acts to reduce expression of co-stimulatory molecules on that APC. This makes it more difficult for the APC to activate potentially self-reactive effector T cells which could recognize that same self antigen.
In the last lecture, you met another type of regulatory T cell: the inducible regulatory T cell. Both inducible and natural regulatory T cells express the Foxp3 protein, but the targets of their suppressive activities appear to be different. Whereas the role of natural regulatory T cells is to provide protection against T cells which have the potential to react against self antigens and cause autoimmunity, the main function of inducible regulatory T cells is to keep the immune system from overreacting to foreign invaders.
Although there is a lot to be discovered about natural Tregs, it is clear that they play an important role in protecting us from autoimmune disease. Indeed, humans who have mutations that compromise the function of the Foxp3 protein suffer from aggressive autoimmune disease and die at an early age.
PERIPHERAL TOLERANCE INDUCTION
Of course, virgin T cells aren't perfect, and some do stray from the prescribed traffic pattern and venture out into the tissues. Indeed, potentially self-reactive T cells are found in the tissues of every normal human. There these "lawbreakers" may encounter self antigens that were too rare in the thymus to trigger deletion, but which are abundant enough in the tissues to activate these T cells. To deal with this situation, there is another level of protection against autoimmunity: peripheral tolerance induction.
Because of the two-key requirement for T cell activation, virgin T cells must not only encounter enough presented antigen to cluster their receptors, they must also receive co-stimulatory signals from the cell that is presenting the antigen. That's where activated antigen presenting cells come in. These special cells have lots of MHC molecules on their surface to present antigen, and they also express co-stimulatory molecules such as B7.
In contrast, ordinary cells such as heart or kidney cells generally don't express high levels of MHC proteins or don't express co-stimulatory molecules, or both. As a result, a virgin T cell with receptors that recognize a kidney antigen could probably go right up to a kidney cell and not be activated by it. In fact, it's even better than that. When a virgin T cell recognizes its cognate antigen presented on a cell, but does not receive the required co-stimulation, that T cell is "neutered." It looks like a T cell, but it can no longer perform. Immunologists say the cell is anergized. In many cases, cells that are anergized eventually die, so peripheral tolerance induction can result in either anergy or death. Consequently, the requirement for the second, co-stimulatory "key" during T cell activation protects us against virgin T cells that venture outside their normal traffic pattern.
TOLERANCE DUE TO ACTIVATION-INDUCED CELL DEATH
Okay, so what if a T cell escapes deletion in the thymus, breaks the traffic laws, and ventures out into the tissues. And suppose that this T cell just happens to find its cognate antigen displayed by MHC molecules at a high enough density to crosslink its receptors on a cell that just happens to be able to provide the co-stimulation required to activate the T cell. What then? Well, all is not lost, because there is yet another "layer" of tolerance induction that can protect us in this unlikely situation.
In the last lecture, we discussed activation-induced cell death (AICD) as one way T cells are eliminated when an invader has been vanquished. This same mechanism also helps protect against virgin T cells that break the traffic rules and are activated by self antigens out in the tissues. T cells in this situation are stimulated over and over by the ever-present self antigens, and when this happens, the self-reactive T cells usually are eliminated by activation-induced cell death. It is as if the immune system senses that this continual reactivation "ain't natural," and does away with the offending, self-reactive T cells.
So T cell tolerance induction is a multilayered process. Instead of trying to test every single T cell for self-reactivity, the immune system employs at least five tolerance-inducing mechanisms. This multilayered approach insures that, for most humans, autoimmune disease never happens.
B CELL TOLERANCE
Immunologists once thought that it might not be necessary to delete B cells whose receptors recognize self antigens. The idea was that the T cells which were needed to help activate potentially self-reactive B cells would already have been killed or anergized. Consequently, B cell tolerance might be "covered" by T cell tolerance. However, it is now clear that mechanisms also exist for tolerizing those B cells which have the potential to be self-reactive.
Most B cells are tolerized where they are born – in the bone marrow, and the antigens that developing B cells encounter in the bone marrow are almost exclusively self antigens. This testing for tolerance of self is the rough equivalent of thymic tolerance induction for T cells. After B cells mix and match gene segments to construct the genes for their receptors, they are tested to see if these receptors recognize antigens that are present in the bone marrow. If its receptors do recognize a self antigen, a B cell is given another chance to rearrange its light chain genes and come up with new receptors that don't bind to a self antigen. This attempt at "redemption" is called receptor editing, and, in mice, at least 25% of all B cells take advantage of this "second chance." Nevertheless, even when they try again to produce acceptable receptors, only about 10% of all B cells pass the tolerance test.
The rest die in the bone marrow.
After testing, B cells with receptors that do not bind to self antigens which are abundant in the bone marrow are released to circulate with the blood and lymph. Of course, induction of B cell tolerance in the bone marrow has the same problems as T cell tolerance induction in the thymus: B cells which have receptors that recognize self antigens that are rare in the marrow can slip through the cracks. Fortunately, bone marrow contains mostly the same abundant self antigens that are found in the secondary lymphoid organs where virgin B cells will be activated. Consequently, self antigens that are too rare to efficiently delete B cells in the bone marrow usually are too rare to activate these B cells in the secondary lymphoid organs. So the traffic pattern of virgin B cells, which restricts them to circulating through the secondary lymphoid organs, helps protect them from encountering abundant self antigens that are not present in the bone marrow.
There also are mechanisms which can tolerize B cells that break these traffic laws. For example, virgin B cells that venture into the tissues can be anergized or deleted if they recognize their cognate antigen but do not receive T cell help. Thus, B cells are subject to mechanisms which enforce self tolerance out in the tissues that are similar, but not identical, to those which tolerize T cells.
MAINTENANCE OF B CELL TOLERANCE IN GERMINAL CENTERS
In contrast to T cells, which are stuck with the same receptors they express when they are tested in the thymus, B cells have a chance, after they have been activated in the secondary lymphoid organs, to modify their receptors through somatic hypermutation. So you may be wondering whether B cells undergoing somatic hypermutation might end up with receptors that can recognize self antigens. If so, these B cells might produce antibodies that could cause autoimmune disease. Fortunately, it turns out that this usually doesn't happen. Here's why.
If a B cell hypermutates in a germinal center so that its receptors recognize a self antigen, it is very unlikely to find and be stimulated by that self antigen advertised on follicular dendritic cells. After all, FDCs only display antigens that have been opsonized – and self antigens usually aren't opsonized. So the first difficulty that potentially self-reactive B cells face in a germinal center is the lack of opsonized self antigen on follicular dendritic cells. But they have another problem – lack of co-stimulation.
After follicular helper T cells have been activated in the T cell zones of secondary lymphoid organs, they move to the lymphoid follicles to give help to B cells. This help takes place during a dance in which follicular helper T cells (Tfh cells) and B cells stimulate each other. The B cells
that participate in this dance have internalized the antigen to which their receptors bind and they use their class II MHC molecules to present this antigen to Tfh cells. The B cells also provide the co-stimulation (e.g., B7) required for the Tfh cell to remain active. In return, the Tfh cell provides the B cell with the co-stimulation (e.g., CD40L) it needs. The important point here is that for this bidirectional stimulation to work, the Tfh cell's receptors and the B cell's receptors must recognize same antigen – or more precisely, parts of the same antigen. If a B cell hyper-mutates so that its BCRs bind to, internalize, and present a self antigen, that new antigen will not be recognized by the "needy" Tfh cell's receptors. As a result, the B and T cells will not be able to collaborate to stimulate each other.
They will have lost their "common interest." And because B cells require Tfh cell help to survive in the germinal center, the interdependence of B and Tfh cells keeps B cells "on track" as they undergo somatic hypermutation.
So self tolerance is preserved during B cell hypermutation for two reasons: the lack of opsonized self antigen required for efficient BCR signaling, and the lack of germinal center Tfh cells which can provide help for B cells that recognize self antigen.
POSITIVE SELECTION OF NATURAL KILLER CELLS
Many viruses try to evade the immune system by down-regulating expression of class I MHC molecules on infected cells. This dirty trick is designed to prevent killer T cells from "looking into" these cells and determining that they are infected. To counter this ploy, natural killer cells survey the cells they come in contact with, and destroy those which do not display class I MHC molecules on their surface – a process called missing self recognition.
This works because NK cells have "inhibitory receptors" on their surface which can recognize class I MHC molecules on healthy cells, and convey a "don't kill" signal so that the potential target cell is spared. But there is a potential problem. MHC molecules are extremely polymorphic (i.e., each MHC gene has many slightly different forms in the human population). Consequently, it is possible that the inhibitory receptors on my NK cells might not recognize my own class I MHC molecules. And if that happened, my NK cells might think that my normal cells were virus-infected and kill them. Not good.
I suppose one solution to this problem would be to co-express each class I MHC molecule with its matching NK inhibitory receptor – but that turns out not to be the way it is done. Rather, every human has multiple genes for inhibitory NK receptors, and these genes also are quite polymorphic. As a result, every person inherits a collection of inhibitory receptor genes, and these usually are different from person to person. Moreover, because these genes seem to be selected at random for expression, the array of different inhibitory receptors actually differs from cell to cell within the same person.
The current thinking is that before NK cells can be fully functional (i.e., deadly), they must be "licensed to kill." To get this license, our NK cells must have inhibitory receptors that can recognize at least one of the class I MHC molecules on our cells. Those NK cells which do not have inhibitory receptors that bind to self MHC molecules are anergized so that they cannot function.
In this way, NK cells are screened to avoid NK cell-mediated autoimmunity. So how are NK cells screened for self tolerance, and where in the body does this "examination" take place? Nobody knows for sure.
There is still a lot to be discovered about how our immune system works!
REVIEW
In this lecture, we discussed one of the most important riddles in immunology: How can the same T cell receptor mediate positive selection (MHC restriction), negative selection (tolerance induction), and activation? The current thinking is that in the thymus, positive selection (survival) of a T cell results from a relatively weak interaction between the cell's TCRs and MHC–self peptides displayed on cortical thymic epithelial cells. This "test" is intended to focus the attention of T cells on antigen presented by MHC molecules, insuring that recognition is restricted to presented antigen, not "native" antigen.
Negative selection (death) of a T cell in the thymus is caused by a strong interaction between the cell's TCRs and MHC–self peptides expressed on medullary thymic epithelial cells or thymic dendritic cells. This "exam" is designed to eliminate T cells which might cause autoimmune disease. Finally, after it leaves the thymus, the T cell can be activated to defend us against disease through a strong interaction between its TCRs and MHC–peptides displayed by professional antigen presenting cells.
Although thymic (central) tolerance induction is pretty good, it isn't perfect. One way of dealing with T cells that escape deletion in the thymus is to restrict the trafficking of virgin T cells to blood, lymph, and secondary lymphoid organs. T cells with receptors which recognize antigens that are abundant in the secondary lymphoid organs usually are efficiently deleted in the thymus – where the same antigens also are abundant. Conversely, self antigens that are rare enough in the thymus to allow self-reactive T cells to escape deletion usually are also too rare to activate virgin T cells in the secondary lymphoid organs. Thus, because of their restricted traffic pattern, virgin T cells normally remain functionally ignorant of self antigens that are rare in the thymus.
Natural regulatory T cells in the secondary lymphoid organs also provide protection against autoimmunity, probably by interfering with the activation of potentially self-reactive T cells. And in those cases where virgin T cells do venture outside the blood–lymph–secondary lymphoid organ system, they generally encounter self antigens in a context that leads to anergy or death, not activation. Moreover, those rare T cells that are activated by recognizing self antigens in the tissues usually die from chronic re-stimulation.
Whereas T cells have a separate organ, the thymus, in which central tolerance is induced, B cells with receptors that recognize abundant self antigens are eliminated where they are born – in the bone marrow. During this screening, self-reactive B cells are given a second chance to "edit" their receptors in an attempt to come up with BCRs that do not recognize self antigens.
As with T cells, tolerance induction in B cells is multilayered. Virgin B cells mainly travel through the blood, lymph, and secondary lymphoid organs. So like T cells, the traffic pattern of naive B cells usually protects them from contact with abundant self antigens on which they were not tested during tolerance induction in the bone marrow. Naive B cells that wander out of the blood/lymph traffic pattern usually don't encounter sufficient self antigen in a form that can crosslink their BCRs. In addition, virgin B cells whose receptors are crosslinked by self antigen in tissues usually don't receive the co-stimulatory signals required for activation – and crosslinking without co-stimulation can anergize or kill B cells.
When B cells mature in germinal centers, they can undergo somatic hypermutation to refi ne the affinity of their receptors. This process creates the possibility that the mutated BCRs might recognize a self antigen. Fortunately, this usually isn't a problem. In order for B cells to proliferate in germinal centers, their receptors must recognize opsonized antigen displayed by follicular dendritic cells – and self antigens normally are not opsonized. Even more importantly, follicular helper T cells in the germinal center will not recognize the self antigen which the mutated BCRs now recognize and present. And B cells count on help from Tfh cells for survival.
The picture you should have is that none of the mechanisms for tolerizing B or T cells is foolproof – they all are a little "leaky." However, because there are multiple layers of tolerance-inducing mechanisms to catch potentially self-reactive cells, the whole system works very well, and relatively few humans suffer from serious autoimmune disease.
Natural killer cells also are tested to avoid autoreactivity.
If an NK cell does not have inhibitory receptors that recognize at least one of a person's class I MHC molecules, that NK cell is rendered non-functional. This process prevents NK cells from attacking healthy cells and causing autoimmunity.


