第11讲 The Intestinal Immune System
HEADS UP!
The intestines are home to trillions of bacteria, some of which "leak" into the surrounding tissues. If the intestinal immune system which protects these tissues reacts too strongly to these bacteria, intestinal disorders can result. On the other hand, if the immune response is too weak, there is the likelihood of a severe bacterial infection. How does the intestinal immune system know whether to respond gently or forcefully?
INTRODUCTION
One of the most interesting aspects of immunology is that there are still many concepts which are not fully understood – or not understood at all! In this lecture, I want to introduce you to an important area about which there are probably more unknowns than knowns: intestinal immunity. This topic will also give us a chance to review some of the concepts we discussed in earlier lectures.
The gastrointestinal system and its role in human health is currently a hot topic in multiple disciplines. This is because it is now recognized that many diseases, such as diabetes, allergies, obesity, some cancers, and inflammatory bowel disease (ulcerative colitis and Crohn's disease), result, at least in part, from an imbalance in the number or type of microbes present in our intestines – or from the immune system's misguided response to these microbes.
The collection of all the microbes (bacteria, viruses, fungi, and parasites) that inhabit our intestines is called the intestinal microbiota. By far the most numerous constituents of the intestinal microbiota are the bacteria. Most of the research done to try to understand the interaction between the microbiota and the immune system involves bacteria, so we will focus on these microbes in this lecture.
Our intestines are home to about 100 trillion bacteria of at least 1,000 different types. Most of these are commensal bacteria (from the Latin, meaning roughly "to eat at the same table"). Commensals are important for our digestion because they produce enzymes which can break down complex carbohydrates in the food we eat – carbohydrates that cannot be dismantled by enzymes made by human cells. Some commensal bacteria also produce vitamins that we require for survival. Moreover, because these "friendly" bacteria are so well adapted to live in our intestines, they help protect us from pathogenic bacteria by out-competing the bad guys for available resources and physical niches. In a sense, the commensals are our microbial "partners."
Although commensal bacteria can have a beneficial, symbiotic relationship with their host, they also can pose a problem. The single layer of epithelial cells which separates them from the tissues that surround the intestines is so thin, its area so vast, and the bacteria so numerous that, even under normal conditions, some of the intestinal bacteria will breach this barrier and enter the tissues.
In fact, the epithelial "barrier" inhibits, but does not prevent, microbes from entering the tissues that underlie the intestines.
This situation poses a real dilemma. If the intestinal immune system were to react too strongly to commensals, the tissues surrounding your intestines would be in a constant state of inflammation – which would cause diarrhea and all sorts of other problems. However, if these errant commensal bacteria were simply ignored, they might enter the blood stream and cause a life-threatening systemic infection. So the intestinal immune system cannot just ignore commensal bacteria. Moreover, pathogenic bacteria, which are not so friendly, also can breach the intestinal barrier. In those situations, the immune system must respond appropriately against these dangerous invaders. What this means is that the intestinal immune system has a unique challenge: It must deal gently with intestinal bacteria that are not inherently dangerous, but harshly with those bacteria which can do us serious harm. How the immune system tells friend from foe and avoids overreaction is currently the subject of intense investigation.
INTESTINAL ARCHITECTURE
To appreciate what the intestinal immune system is up against, we need to have a clear picture of the digestive system and how it works. It is important to note that topologically, our gastrointestinal tract is actually part of the "outside environment." It is essentially a tube that runs though our body from "top to bottom." Here is a schematic representation which shows the basic layout.
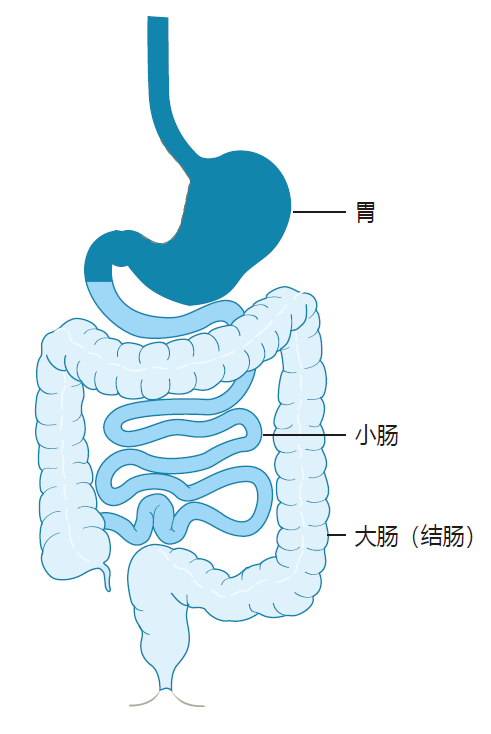
Most of the action, so far as the immune system is concerned, takes place below the stomach in the small intestine and the large intestine (colon). The primary function of the small intestine is digestion, and the requirement for absorption of nutrients dictates that the small intestine must have a large area. Indeed, the small intestine of a human is about 6 meters in length, and its epithelial surface includes millions of finger-like projections called villi, which expand the total surface area of the small intestine to nearly 200 square meters. In contrast, the large intestine is only about 1.5 meters long, has no villi, and plays almost no role in digestion. Its primary function is the absorption of water and salt from the intestinal contents. Importantly, the large intestine is home to the vast majority of the commensal bacteria that inhabit the digestive tract.
The lumen (i.e., the inside) of both the large and small intestine is bounded by a single layer of epithelial cells.
These cells stand shoulder-to-shoulder, are joined together by tight-junction proteins, and are coated with protective mucus which is generated by goblet cells that are part of the epithelium. The epithelial cell layer is renewed every four or five days, and it separates the contents of the intestines from the tissues that surround the intestines called the lamina propria.
The protective mucus in the small intestine is only one layer thick and rather porous – which is important for the efficient uptake of nutrients. Fortunately, the food and bacteria we ingest move rapidly through the small intestine, so bacteria have to work fast if they are going to get a foothold there. Moreover, the mucus is rich in antibacterial proteins such as lysozyme, which are secreted by cells in the epithelium, and which can attack the membranes which surround some bacteria. Here is a diagram that shows important features of the small intestine.
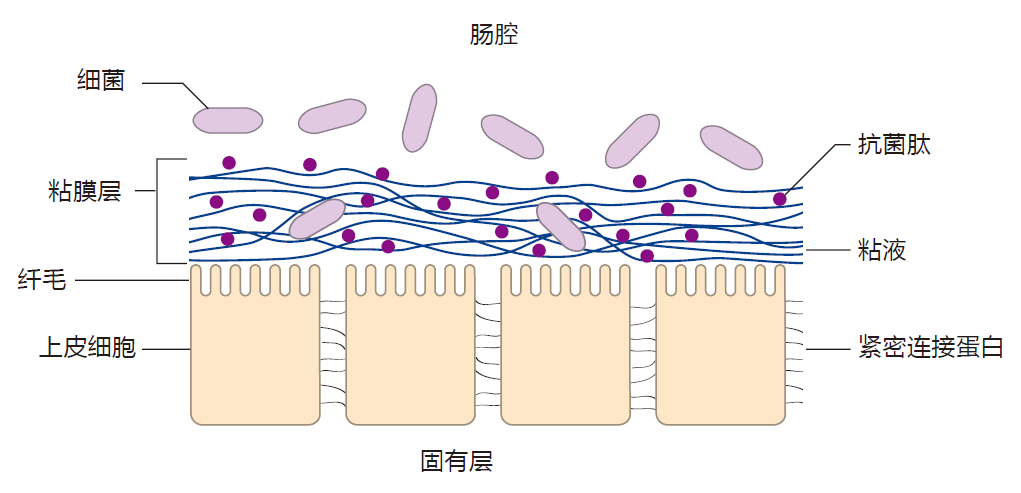
The epithelium of the large intestine is protected by two layers of mucus. The inner layer is firmly attached to the epithelium, and is rather like a pad of steel wool. This layer of mucus is relatively bacteria-free, and is rich in antimicrobial peptides (e.g., α-defensins). On top of this dense inner pad is another layer of mucus which, like the single layer in the small intestine, is less dense and more like a slimy net.
The intestinal mucus has several important functions.
It acts as a diffusion barrier which denies most of the bacteria in the lumen access to the epithelium. The mucus also concentrates antimicrobial proteins near the epithelial surface – antimicrobials which can destroy bacteria that might try to breach this barrier. These features are important because intestinal infections usually begin when invaders adhere to the epithelial cells that line the intestine. The goblet cells which produce the mucus are hard workers, and the mucus is replaced in a matter of hours. As a consequence, bacteria that are trapped in the mucus are rapidly shown out the "back door" – if you know what I mean.
The mucin proteins that make up the mucus are highly glycosylated. Commensal bacteria feast on these attached carbohydrates, and convert them into short chain fatty acids such as butyrate and acetate. These molecules easily diffuse through the mucus, and provide an important energy source for the cells of the epithelium.
CHALLENGES FACED BY THE INTESTINAL IMMUNE SYSTEM
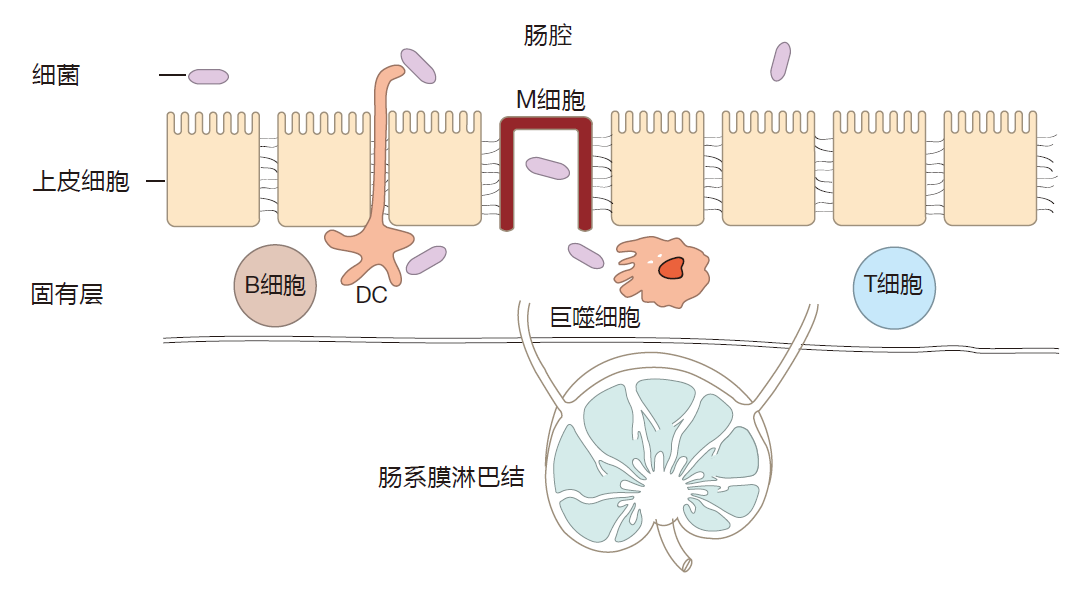
Now that we have an understanding of the architecture of the intestines, we can discuss how the immune system deals with bacteria which "wander" out of the intestinal lumen and into the tissues. One of the defining characteristics of commensal bacteria is that although they may adhere to the epithelium, they do not actively cross this intestinal barrier. Nevertheless, commensals do make their way into the lamina propria as the result of small breaks in the epithelial barrier (no barrier is perfect), and this happens almost continuously. Moreover, after they adhere to the epithelium, some pathogenic bacteria produce virulence factors which allow them to cross the barrier and enter the lamina propria. So the picture you should have is that the intestinal immune system is under constant attack by bacteria and other invaders.
Commensal or pathogenic bacteria which breach the epithelial barrier usually are intercepted by resident macrophages – the most abundant immune system cell in the lamina propria. Invading bacteria also can be transported to nearby mesenteric lymph nodes by the lymphatic vessels that drain the lamina propria. And during an intestinal invasion, dendritic cells which reside in the tissues that surround the intestines can travel via the lymphatic route to mesenteric lymph nodes. There they can activate T cells which are specific for the invader, and encourage these effector cells to travel back to the lamina propria to do battle with the enemy.
Now if this were all there was to the intestinal immune system, we'd be in big trouble. Commensals are continually breaching the epithelial barrier, so our intestines would be in a state of constant war. Instead of that single splinter in your big toe, this situation would be the rough equivalent of having bacteria-laden splinters piercing the skin all over your body, all the time. It would be awful – and lethal!
RESPONDING GENTLY TO LIMITED THREATS
Clearly, there must be special features of the intestinal immune system which are different from the "systemic" immune system that protects the rest of our body. Let's see what they might be.
An anti-inflammatory environment
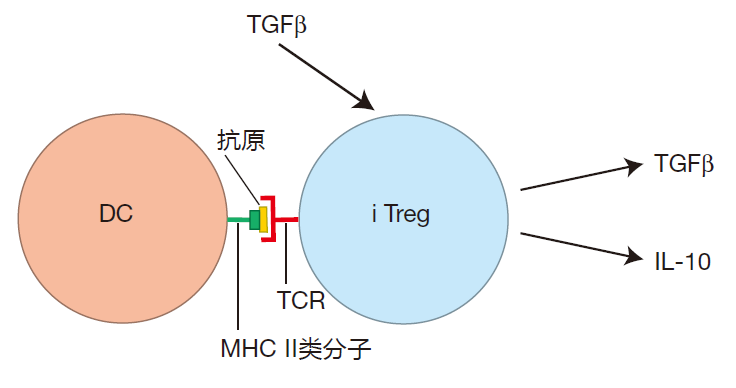
In contrast to the systemic immune system, where inflammation is the game, the "default option" for the intestinal immune system is anti-inflammatory. Indeed, under normal conditions, the environment surrounding the intestines is heavily biased toward producing a gentle reaction. In Lecture 8, we discussed inducible regulatory T cells – special Th cells whose job is to limit inflammation. It turns out that the lamina propria is home to a large number of these cells. The reason for this is that healthy intestinal epithelial cells produce TGFβ, a cytokine which encourages Th cells that are activated in the intestinal environment to become iTregs. These T cells then give off cytokines such as TGFβ and IL-10, which help "calm down" the mucosal immune system. In addition, many CTLA-4 checkpoint proteins are expressed on the surface of iTreg cells. These CTLA-4 proteins can bind to and "mask" the B7 proteins on antigen presenting cells in the lamina propria, thereby decreasing the APC's ability to activate effector T cells.
In some cases, commensal bacteria contribute directly to help maintain the normally immunosuppressive environment of the lamina propria. For example, as part of their normal metabolism, some commensal bacteria produce butyrate. This short-chain fatty acid influences Th cells in the lamina propria to become regulatory T cells, which secrete "calming" cytokines. Likewise, Bacteroides fragilis, a commensal bacterium, produces a molecule called polysaccharide A. When Toll-like receptors on helper T cells in the lamina propria detect this polysaccharide, those T cells are instructed to produce IL-10, which dampens inflamation. Bifidobacterium is a commensal which is a common constituent of the probiotics many people take to "promote intestinal health." When the Toll-like receptors of intestinal dendritic cells detect the presence of Bifidobacterium breve, those DCs are prompted to produce IL-10 to calm the intestines.
Non-inflammatory macrophages In response to an infection, the normal job of a macrophage is to cause inflammation. For instance, when the tissues beneath the skin are infected by bacteria, macrophages not only phagocytose these invaders, they also secrete cytokines which alert other immune warriors and summon neutrophils from the blood to join in the battle.
The result is inflamed tissues at the site of the invasion.
Fortunately, the IL-10 produced by iTregs in the lamina propria encourages macrophages that patrol this area to be "non-inflammatory." What this means is that although these macrophages are highly skilled at phagocytosis, they don't give off cytokines which would signal a full-blown attack and cause inflammation. Consequently, non-inflammatory macrophages can deal gently either with the small number of commensals which continually "leak" from the intestines into the lamina propria or with a small attack by pathogenic bacteria.
IgA antibodies
IgA is the major antibody class produced by B cells in the lamina propria. In fact, IgA is an antibody which evolved especially for the protection of mucosal surfaces. Some of the IgA antibodies produced by lamina propria B cells are transported through the epithelial cells (are "transcytosed") and are released into the lumen of the intestines.
This "secretory" IgA binds to microbes there and prevents them from adhering to the epithelial cells that line the intestine. Indeed, most bacteria in the colon are coated with IgA antibodies. And because the intestinal mucus is renewed frequently, clumps of IgA-bound microbes can be rapidly eliminated with the feces. So the main task of secretory IgA is exclusion.
In addition to helping prevent luminal bacteria from crossing the epithelial barrier, IgA antibodies made by lamina propria B cells also can intercept invaders once they have breached the intestinal barrier and have entered the lamina propria. IgA antibodies in the lamina propria can bind to invaders, transcytose epithelial cells with their cargo, and usher the intruders back out into the intestine for disposal. Importantly, secretory IgA does not cause inflammation. This is because the Fc portion of this antibody cannot bind to receptors on immune system cells to trigger an inflammatory response – as IgG antibodies would do. Consequently, IgA antibodies can deal gently with intestinal invaders without causing inflammation.
It is not entirely clear how B cells in the lamina propria are influenced to make the IgA class of antibodies, and not, for example, IgG antibodies. It is known that retinoic acid produced by intestinal dendritic cells can drive IgA production. Retinoic acid also imprints IgA-secreting plasma B cells with an "intestinal identity," so that they travel back to the tissues which surround the intestines.
In most cases, class switching requires the help of Th cells.
This assistance involves the ligation of CD40 on the B cell surface by CD40L on the surface of a helper T cell. However, it has been discovered that B cells of the intestinal immune system actually can switch to the production of IgA antibodies without T cell help. It is presumed that other proteins in the intestinal environment substitute for CD40L, and ligate the CD40 proteins on intestinal B cells.
But there is a lot of mystery surrounding these special, IgA-producing, lamina propria B cells.
A distributed response The systemic immune system is designed to respond "locally." For example, if you have a splinter in your big toe, B and T cells activated in the lymph nodes that drain the toe will recirculate through the lymph and blood, and exit the blood stream where the battle is being waged in your toe. After all, these weapons are specific for the particular invader that has attacked your big toe today, so it wouldn't be useful to send them to your calf – or even to your little toe. There is nothing going on there. The intestinal immune response is quite different.
Although B and T cells might be activated in response to bacteria that entered the lamina propria 1 meter down in your small intestine, those lymphocytes don't return just to that spot. In fact, they are distributed throughout the lamina propria. Why is this, you may ask? Doesn't that seem wasteful?
The answer is that whereas the splinter piercing your toe is a rare event, invasions by the resident bacteria in your intestines are continual. Moreover, although the types of commensals do vary as one goes from the top of the small intestine to the anus, the same commensals are present over long stretches of the intestines. Consequently, a distributed response, in which B and T cells specific for intestinal invaders are stationed throughout the lamina propria, makes sense. This distributed response has another important feature. In the big toe example, it takes some time to mobilize the troops that are specific for that invader, and to deliver them to the battleground. In contrast, the intestinal immune system is "prepared in advance" to deal with common invaders because lymphocytes and IgA antibodies are already "on site." The result is a lightning fast response that can deal with attackers before they can multiply in the tissues, thereby limiting the amount of inflammation.
THE INTESTINAL IMMUNE SYSTEM'S RESPONSE TO PATHOGENS
Okay, so the intestinal immune system is set up to provide a gentle response to commensal bacteria and to small numbers of pathogens. However, in large numbers, both commensals and pathogenic bacteria can cause damaging infections. So how does the intestinal immune system deal with these dangerous situations?
In response to serious attacks, Th1 cells can be activated. These helper cells encourage the production of IgG antibodies, and Th1 cells secrete cytokines such as IFN-γ which enhance the killing power of lamina propria macrophages. Also, when helper T cells are activated in an environment that is rich in TGFβ and IL-6 or TGFβ and IL-23, these cells are influenced to become Th17 cells – helper T cells which play an important role in the intestinal immune defense against dangerous attacks.
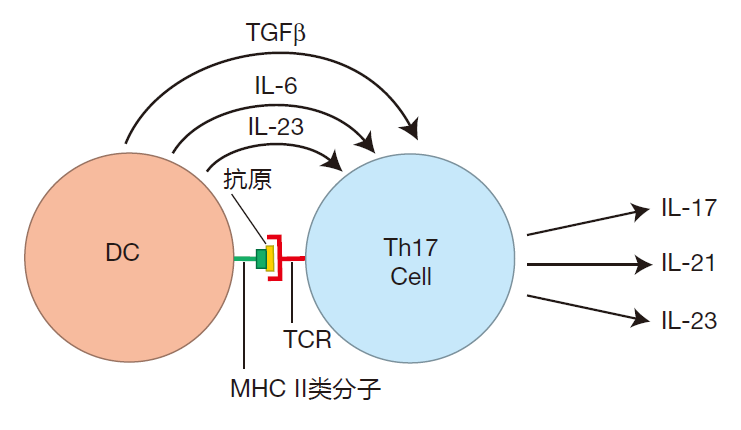
Th17 cells are highly inflammatory. The "signature cytokine" they produce, IL-17, recruits huge numbers of neutrophils from the blood stream – warriors which are just the ticket for dealing with a dangerous bacterial invasion. Cytokines secreted by Th17 cells also function to increase the effectiveness of the intestinal barrier by strengthening the tight junctions between epithelial cells.
In addition, these cytokines stimulate antimicrobial peptide and mucus production by intestinal epithelial cells, and act to facilitate the transcytosis of IgA antibodies and their cargo out into the intestinal lumen.
Another important feature of the intestinal immune system is that it is designed to function independently of the immune system in the rest of the body. Dendritic cells that are activated in the lamina propria travel to the mesenteric lymph nodes that drain the intestinal tissues – but they do not travel any farther along the chain of lymph nodes.
In addition, B and T cells activated in the mesenteric lymph nodes have strict instructions to take up residence in the lamina propria. They do not enter the normal traffic pattern of circulating lymphocytes, which would carry them to other parts of the body. So the intestinal immune system is a "private" system. What happens in the intestinal immune system usually stays in the intestinal immune system.
HOW TO RESPOND?
So the intestinal immune system can respond gently to attacks that are not serious, and it has the "tools" to deal harshly with dangerous pathogens that invade via the digestive tract. But how does the intestinal immune system know to react gently to small doses of commensals
or pathogens, and vigorously when there is real danger?
This is the central question that immunologists who study the intestinal immune system are asking.
TGFβ is a cytokine that drives helper T cells to become iTregs – which are anti-inflammatory. However, TGFβ also is one of the cytokines that causes naive Th cells to become Th17 cells – cells which are skilled at orchestrating an inflammatory response to a bacterial or fungal invasion. So how does the immune system decide whether Th cells should become iTregs, and restrain the immune response, or become Th17 cells, and "let the dogs out"? The complete answer is unknown. However, as you might predict, dendritic cells in the lamina propria are thought to play a critical role in maintaining the proper balance between a gentle or an inflammatory response.
Dendritic cells in the Peyer's patches of the small intestine intercept luminal antigens which have been delivered into the lamina propria by transcytosis through the M cells that crown these patches. In addition, some lamina propria DCs can extend their dendrites between the epithelial cells to make direct contact with antigens in the intestinal lumen. Using these mechanisms, DCs deliberately and continuously sample what is going on in the intestines, and use this information to decide on an appropriate course of action.
Dendritic cells are equipped with pattern-recognition receptors that can recognize bacterial "signatures."
Some of the most pathogenic intestinal bacteria (e.g., Salmonella) are equipped with flagella, which help them "swim" through the mucus so they can access the intestinal epithelium. The flagellin protein, from which flagella are constructed, can be detected as a danger signal by pattern-recognition receptors called TLR5 on the surface of intestinal dendritic cells. And when their pattern-recognition receptors detect flagellin, DCs begin to produce IL-6, which instructs Th cells to become Th17 cells.
So, if there is no real danger, and things just need to be kept calm, lamina propria DCs don't produce IL-6, and naive Th cells – under the influence of tissue-produced TGFβ – become iTregs. On the other hand, if there is an invasion of pathogenic bacteria that have flagella, dendritic cells produce IL-6, which causes helper T cells to commit to becoming Th17 cells. One important feature of this iTreg to Th17 "switch" is that iTregs are very short lived. Consequently, the switch from suppression to defense can be made quickly.
It is important to note, however, that commensals and pathogenic bacteria share many of the same molecular features, so in most cases, it is not clear how dendritic cells distinguish between pathogenic and commensal bacteria. It may be that pathogens and commensals
trigger different combinations of pattern-recognition receptors, leading to different outcomes. It also may turn out that the response to pathogens and commensals frequently is the same, and that the decision to respond gently or violently depends on the size of the invasion.
In any case, how the intestinal immune system determines the appropriate response to intestinal invaders is one of the most important unsolved mysteries in immunology. Roughly 1.5 million Americans suffer from Crohn's disease or ulcerative colitis – conditions that are thought to result from an inappropriate inflammatory response to commensal bacteria. It is hoped that a better understanding of the intestinal immune system's decision-making process, and how these decisions are implemented, may lead to improved treatments, or even a cure, for these diseases.
REVIEW
Trillions of intestinal bacteria are separated from the tissues that surround the intestines by a single layer of epithelial cells covered with mucus. Most of these bacteria are commensal bacteria that have evolved a mutually beneficial relationship with their human host. However, there also are pathogenic bacteria which inhabit the intestines, and these can do us serious harm. Both types of bacteria can breach the epithelial barrier, and both must be dealt with by the intestinal immune system.
A variety of immune system defenders, including macrophages, dendritic cells, and lymphocytes, are found beneath the intestinal epithelium in the lamina propria.
Under normal conditions, only small numbers of bacteria leak from the intestines into the lamina propria, and the immune warriors there operate in an environment which encourages them to deal gently with invaders. "Non-inflammatory" macrophages in the lamina propria are highly phagocytic, but they normally do not secrete battle cytokines which would "stir up" a full-blown inflammatory response. B cells in the lamina propria specialize in producing IgA antibodies, which deal passively with invaders by "quietly" transporting them back out into the intestines to be eliminated with the feces. In addition, healthy intestinal epithelial cells produce cytokines which help keep the intestinal immune system relatively calm. These cytokines can induce helper T cells to become regulatory T cells – cells which produce cytokines that have a soothing effect on the immune warriors stationed in the lamina propria.
Dendritic cells in the lamina propria continuously evaluate the danger posed by current invaders. If there is a serious breach of the epithelial barrier, the intestinal immune system can switch rapidly from a gentle response to an aggressive reaction. Alerted dendritic cells can instruct helper T cells to become Th1 or Th17 cells. These helper T cells then orchestrate an inflammatory response in which formerly non-inflammatory macrophages become "angry," and neutrophils are recruited from the blood to engage invaders in hand-to-hand combat.
The weapons of the intestinal immune system are deployed over large areas of the intestines. Because of this distributed response, the intestinal immune system is prepared to deal rapidly with common invaders before they can proliferate to build up their numbers. On the other hand, the intestinal immune system is "private": Intestinal attacks normally are dealt with locally, without spilling over into the rest of the body.
Although some pathogenic bacteria may have unique signatures that alert the intestinal immune system to danger, commensal bacteria and pathogenic bacteria share many of the same molecular features.
Consequently, how the intestinal immune system differentiates between infections that can be dealt with gently and serious infections that must be dealt with harshly is one of the important unsolved mysteries in immunology.


