8.3 CHANGES IN SKIN
Human skin is the largest organ of the body and serves as the boundary between our bodies and the outside world. Our skin not only contains our internal body components but protects them from the sometimes harsh and harmful external environment. For example, harmful solar radiation, such as ultraviolet (UV) light, does not penetrate below the level of human skin. Our skin also participates in the innate immune system (discussed later in this chapter) by establishing a barrier against toxic invasion. In addition to its protective role, skin serves as an organ of the thermoregulatory system and as a sensory organ for touch. In this section, we discuss the basic structure of skin, how aging affects it, and the role of environmental factors in time-dependent changes in the skin. The skin's role in the immune system is briefly described in the later section “Changes in immune system.”
Skin consists of three layers
There are three layers of skin: epidermis, dermis, and subcutaneous fat tissue (Figure 8.21). The epidermis is the outer layer of skin. It consists of five layers: the stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale. Although each layer has a specific function, the overall purpose of the epidermis is to prepare the living skin cells, the keratinocytes, produced in the stratum basale, to become the flat, dead, hard cells of the stratum corneum. This dead skin sheds off about every 2 weeks and is replaced by new dead skin cells that have moved upward through the layers. Through this process, cells damaged by solar radiation or other insults are sloughed off, protecting the underlying layer. The epidermis also contains several specialized cells, including melanocytes, which produce pigments; Langerhans cells, which play a role in the immune system; and Merkel cells, which are a form of sensory receptor.
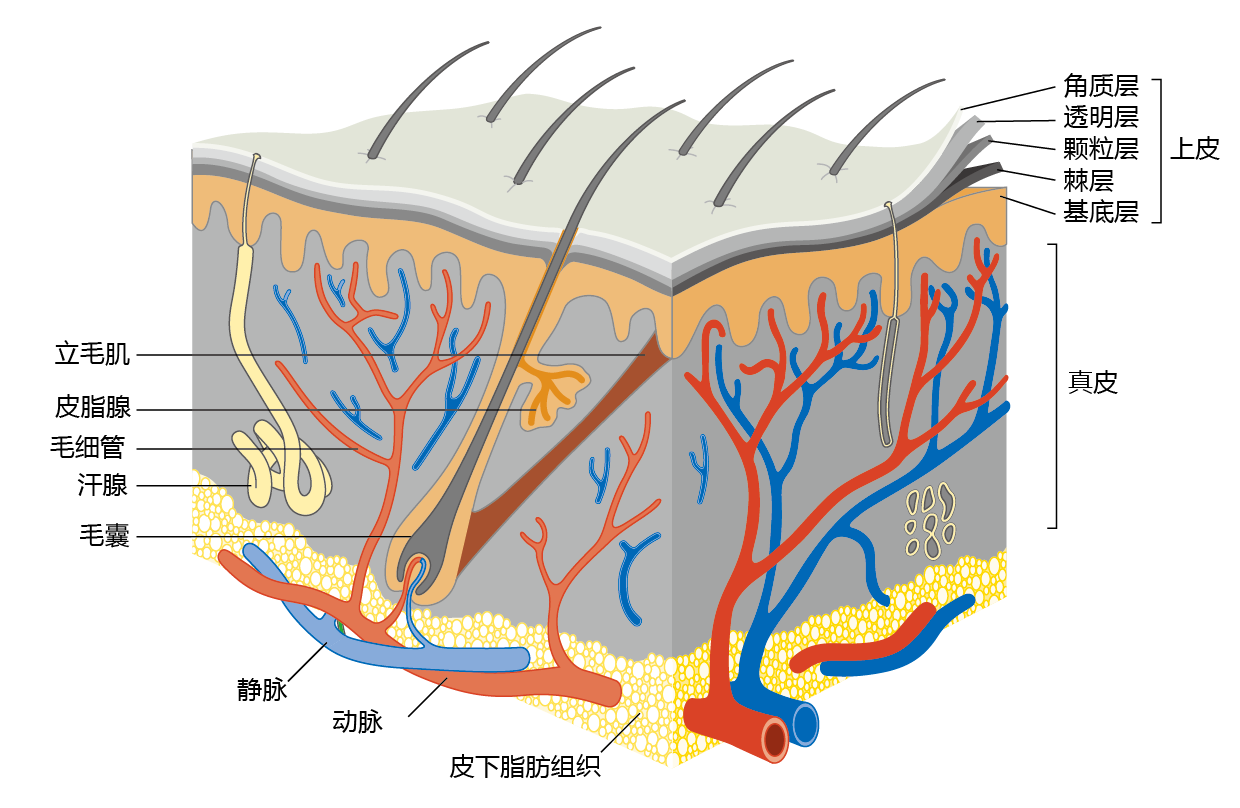
Figure 8.21 Cross-sectional diagram of human skin. The three layers of the skin—epidermis, dermis, and subcutaneous fat tissue—protect the internal organs from environmental insult, provide our sense of touch, and help to thermoregulate the body through sweating (if hot) and vasoconstriction (if cold).
The dermis consists mostly of connective tissue and is much thicker than the epidermis. It contains the organelles of the skin: exocrine (sweat) glands, hair follicles, sebaceous (oil) glands, capillaries, and nerve endings. These structures are supported by a matrix of elastic proteins, collagen, and elastin fibers. (Collagen accounts for about 70% of the dermis's dry weight.) The dermis is responsible for the skin's pliability and helps transmit the sensations of touch and pressure. It is also involved in the regulation of body temperature. The sweat glands release water onto the surface of the skin, allowing evaporation and cooling when the internal body temperature increases. Conversely, during cold exposure, blood vessels in the skin constrict and shunt blood to the internal organs.
Subcutaneous fat tissue, making up the bottom layer of the skin, contains adipose (fat) tissue and blood vessels. The fat layer provides cushioning and insulation. It also helps to regulate the temperature of both the skin and the body.
Wrinkles are caused by loss of skin elasticity and subcutaneous fat
Wrinkles are one of the most visual consequences of aging; they occur in 100% of the human population and in all parts of the body. The underlying cause of wrinkles is a decrease in the number of skin cells developed by the dermis, changes in the normal functioning of the skin cells, and declining subcutaneous fat. The time-dependent decrease in the number of skin cells results in a thinning of the skin and occurs primarily in the epidermis. Cell loss appears to result from a slowdown of cell division caused by the shortening of telomeres (see Chapter 4). Some studies have also found that the number of mitotic cells in the stratum basale declines with age. As a result of the loss in skin cells, production of elastin and collagen decreases, and the skin loses significant elasticity. The skin also loses elasticity due to an increase in nonenzymatic cross-linking in collagen (BOX 8.2). With each new cross-link, the elastic properties of the protein decline and the dermis flattens. With the loss of elasticity, the epidermis no longer smoothly covers the irregularities in the underlying dermis and subcutaneous fat.
| BOX 8.2 THE MAILLARD REACTION AND AGING |
|
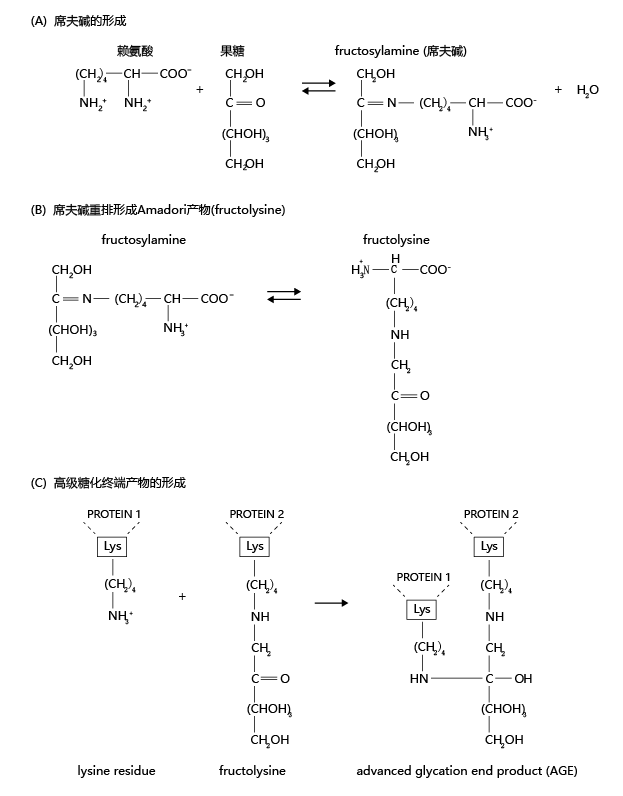
Time-dependent decline in physiological function may be the result of a biochemical rearrangement of proteins that has many similarities to the process that causes foods to turn brown when heated. The biochemical pathway leading to the browning effect of food, known as the Maillard reaction (named after Louis Camille Maillard, who discovered the pathway), causes our arteries to stiffen, increases joint stiffness and decreases joint range of motion, thickens the lens of the eye, and plays a part in many other non-life-threatening changes. Here we briefly discuss the biochemistry of the Maillard reaction and its effect on human aging. The Maillard reaction occurs in three steps. First, glucose or fructose molecules react with an amino acid to form a Schiff base, an organic compound in which the nitrogen atom of an amino group is double-bonded to a carbon atom of glucose or fructose (Figure 8.22). The enzymatic formation and degradation of Schiff bases serves an important synthetic and signaling function in normal biochemistry and is highly regulated. However, Schiff bases can also be formed through nonenzymatic processes, which are not regulated, and can lead to the accumulation of nondegradable cellular metabolites.
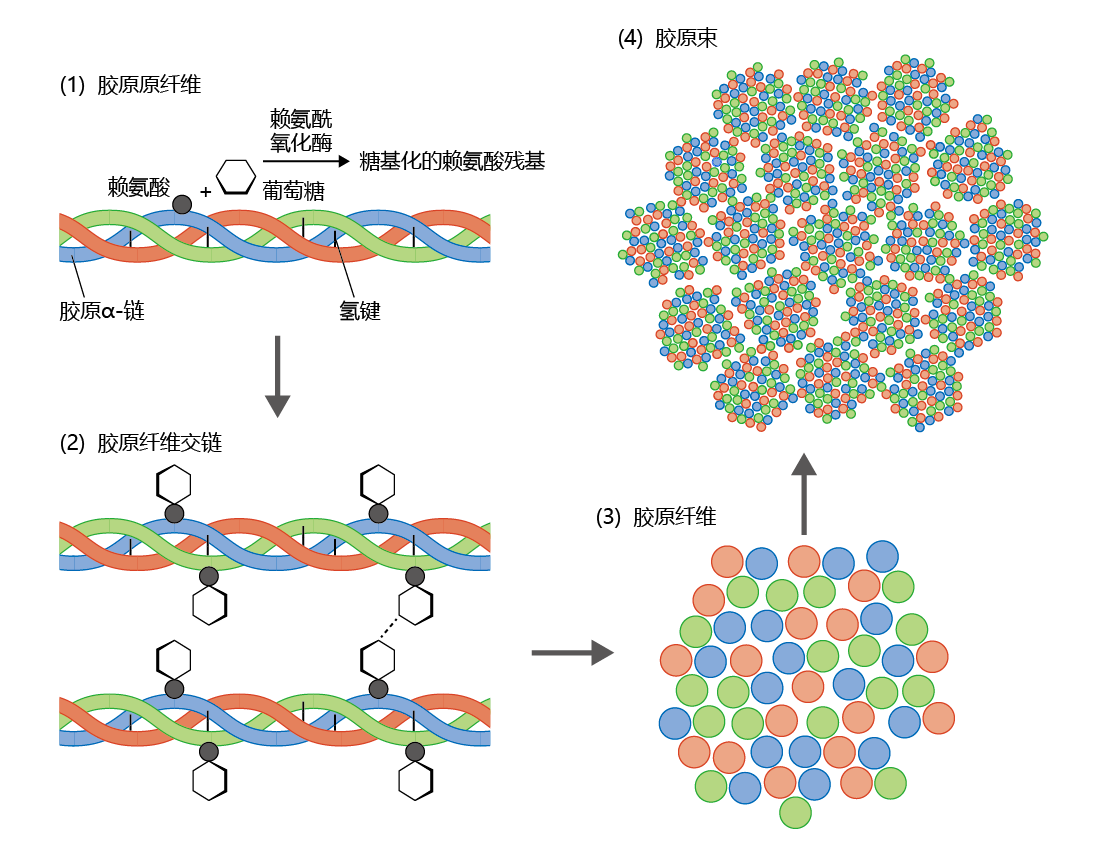
Figure 8.22 The Maillard reaction and the nonenzymatic formation of an advanced glycation end product (AGE). (A) An amino acid, such as lysine, forms a double bond with an aldose, such as fructose, to form a Schiff base (fructosylamine). (B) The Schiff base can undergo rearrangement to form the more stable ketone known as an Amadori product, represented here as fructolysine. Unlike Schiff bases, Amadori products can accumulate in the cell. (C) Over time, the Amadori product attached to one protein molecule (or protein subunit) bonds with a second protein molecule, creating a cross-link and forming an AGE. (The linkage between the two proteins is shown only as a representation and is not intended to be chemically correct.) Formation of the AGE cannot be reversed and may lead to inactivation of the protein. In the second stage of the Maillard reaction, the Schiff base undergoes nonenzymatic rearrangement to form an Amadori product. Amadori products are much more stable than Schiff bases and can accumulate within the cell. The third and final stage of the Maillard reaction results in the formation of an AGE, consisting of two separate protein molecules linked together by the Amadori product. These linkages, called crosslinks, change the protein's structure and thus alter its function. AGEs are highly insoluble and are not easily degraded. When these products are heated, they cause browning. AGEs differ from normal, enzymatically cross-linked products and can result in time-dependent loss of physiological function. For example, collagen makes up more than 25% of the total protein in our bodies and represents the majority of protein in the extracellular matrix, the substance that holds cells together. The collagen fibril consists of three α-chains twisted into a triple helix and held together by hydrogen bonds (Figure 8.23). During translation, as collagen fibrils are synthesized, the enzyme lysyl oxidase catalyzes a reaction that joins a lysine residue in the protein to the aldehyde group of an aldose, usually glucose or fructose, creating a glycosylated lysine residue. The glycosylated lysine residues of several collagen fibrils bond spontaneously to form the collagen fiber. In turn, collagen fibers bind together to form a collagen bundle. The enzymatic bonding of one fibril to another provides collagen with its unique strength and elasticity.
Figure 8.23 Enzymatic cross-linking of the collagen fiber. (1) The collagen fibril, the basic unit of the collagen fiber, contains three α-chains twisted into a helix held together by hydrogen bonds. Lysyl oxidase catalyzes a reaction between a lysine residue in an α-chain and an aldehyde, shown here as glucose, to form a glycosylated lysine residue. (2) The glycosylated lysine residue will bond spontaneously with other glycosylated lysine residues to form cross-links between fibrils. (3) Several cross-linked fibrils make up the collagen fiber. (4) Several collagen fibers bond together to form the final product—the collagen bundle. The formation of collagen occurs with just enough cross-linking to provide sufficient strength while retaining elasticity. (If you pull your skin away from the underlying muscle, it will snap back into place because of the elasticity of collagen.) Collagen turnover is extremely slow, and it is not unusual to find organs in which the collagen laid down during development remains intact throughout the life span. This property of collagen leaves the protein highly susceptible to the random and unregulated process of nonenzymatic glycosylation and the formation of AGEs. Each nonenzymatic cross-link formed in the collagen fiber increases the collagen's strength (the collagen becomes stiffer) and decreases its elasticity (reducing its flexibility). The importance of AGE formation in inactivating proteins cannot be overemphasized. Because AGEs have no known catabolic pathway, the accumulation of these products has been suggested to cause cellular and physiological dysfunction. As presented in this chapter and in Chapter 9, the formation of nonenzymatic crosslinks and AGEs is suggested as a likely contributor to time-dependent physiological decline. |
The most significant cause of the irregularities in skin shape that arise with age is the decline in stored lipids in the subcutaneous fat layer. The majority of body fat in the average adult human is in subcutaneous fat. Young adults (20- to 40-year-olds) tend to store fat equally throughout the body. As we age into the sixth decade and beyond, the fat stores are distributed more to the abdomen (men), hips (women), and buttocks (both sexes). Moreover, and for unknown reasons, subcutaneous fat in the face, arms, and legs decreases. As we age, the loss of subcutaneous fat in these regions causes irregularities that are covered by a relatively inelastic epidermis, thus creating wrinkles.
Ultraviolet light causes significant damage to skin over time
Extrinsic factors such as pollution, tobacco smoke, and excessive alcohol consumption can cause significant damage to the skin. However, more than 90% of the time-dependent environmental damage to skin is caused by sun exposure. Photoaging—the long-term damage to skin caused by UV light—occurs mainly within the dermis and results from an accumulation of abnormal elastic tissue, a condition known clinically as solar elastosis. The accumulation of damaged elastic and collagen fibers results in a dysfunctional extracellular matrix and leads to loss of elasticity of the skin, wrinkle formation, and telangiectasia (appearance on the skin's surface of small dilated blood vessels). In addition, repeated bouts of overexposure to the sun's radiation (i.e., sunburn) damage the melanocytes. Alterations in melanocyte function and structure can lead to a form of skin cancer called melanoma in genetically at-risk individuals. The importance of the ability of melanocytes to produce the protective skin pigment melanin is evident from the fact that dark skin photoages at a much slower rate than lighter skin.
The etiology of photoaging and solar elastosis has yet to be conclusively determined. Most research indicates that the UV-generated role of ROS (also called oxygen-centered free radicals) may have a central role. Recall from Chapter 4 that the unpaired electrons of ROS react quickly with other molecules and alter protein structure and function. Since collagen and elastin have very slow turnover rates, damaged elastic fibers tend to accumulate in the matrix of the dermis. Damage to the elastic fibers also initiates the immune response, which can cause further damage to the dermis. Many investigations have shown that repeated bouts of immune system response to ROS-damaged tissue accelerate the rate of skin aging.
Photoaging can be completely prevented—do not expose yourself to the harmful UV radiation of the sun or tanning booths. However, since many people are unable or unwilling to take this drastic step, limiting the amount of time spent in the sun and using sun block can significantly reduce photoaging and the risk of skin cancer.