8.4 CHANGES IN SENSES: HEARING, VISION, TASTE, AND SMELL
Hearing and vision are two of the few physiological systems that undergo time-dependent declines in the majority of individuals over the age of 70. Neither type of decline, however, necessarily causes significant impairment to daily life, as the medical sciences have developed treatments that effectively negate the time-dependent losses: hearing aids increase volume and change the pitch; reading glasses improve close-up vision. Taste and smell are our two chemical senses. Contrary to popular belief, time-dependent changes in taste buds and olfactory centers are minimal. In this section, we examine how our sensory organs, and therefore our perceptions and interactions with the outside world, can change as we age.
Sense of hearing is based on physics of sound
To understand the physiology of hearing and any time-dependent change that may occur, you must first understand the physics of sound. A sound wave begins when an object vibrates in a medium (gas, liquid, or solid). Remember that vibrations are oscillating in nature. That is, a vibration pushes outward beyond the resting state of the object, causing compression of the molecules in the surrounding medium and an increase in pressure. The outward motion of the vibration then retracts beyond the resting state of the object, causing a decrease in pressure in the medium, or rarefaction.
The height difference between a compression peak and a rarefaction peak defines the amplitude of the sound wave (Figure 8.24). The loudness of a sound is directly proportional to the wave's amplitude. As the sound wave radiates outward from the object creating the vibrations, the amplitude becomes less and the sound softer. The loudness of a sound is measured in decibels. One decibel is the logarithmic increase in loudness above a sound that is barely audible to the human ear. The human ear can tolerate sound between 0 and 130 decibels. Sounds greater than 130 decibels cause pain. The frequency of the vibration, defined as the distance between consecutive peaks of either compression or rarefaction, determines the pitch of the sound: the higher the frequency, the higher the pitch; the lower the frequency, the lower the pitch. Although the human ear can hear sounds between 20 and 20,000 Hz (hertz, or cycles per second), it best detects sounds between 1000 and 4000 Hz.
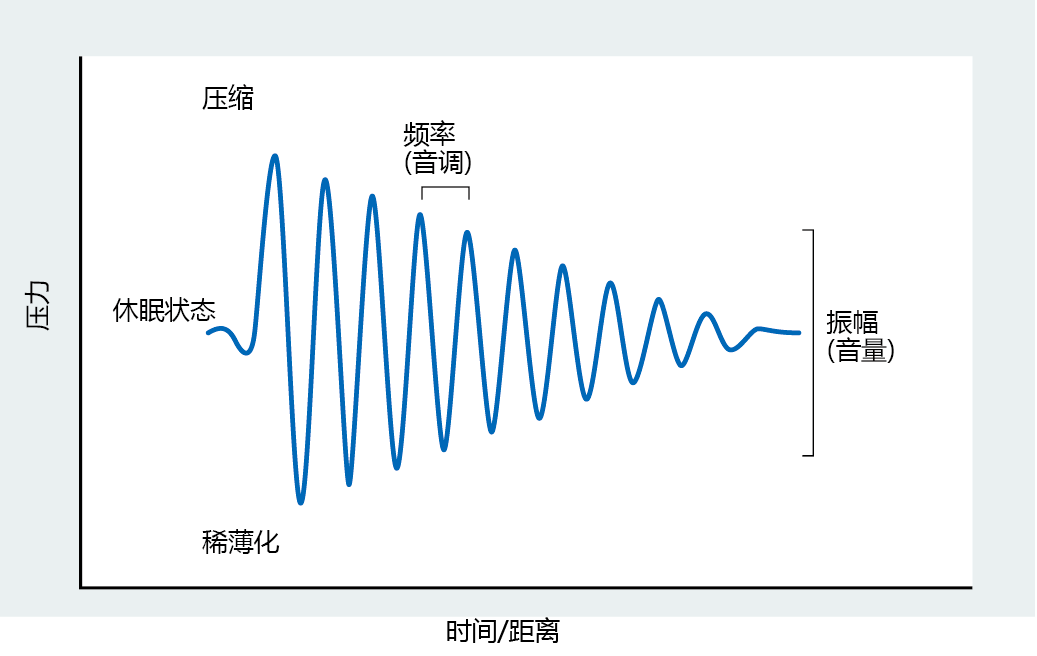
Figure 8.24 Components of a sound wave. The outward push of a vibrating object decreases the distance between molecules in the surrounding medium and increases the pressure (compression). The inward contraction of a vibrating object increases the distance between molecules in the medium and decreases the pressure (rarefaction). The distance between the compression and rarefaction peaks defines the amplitude of the sound wave. Loudness of the sound is directly proportional to amplitude. The frequency of the wave, or how fast the vibrations occur, determines the pitch of the sound.
Transmission of sound through human ear occurs in three steps
To hear sounds, the human ear has to do three things: (1) direct the sound waves to the hearing part of the ear, (2) sense the fluctuations in air pressure caused by the sound vibrations, and (3) translate these pressure fluctuations into a signal that the brain can understand. Each of these tasks is performed by a different part of the ear.
The first step of sound transmission involves the outer ear, comprising the pinna, the external auditory canal, and the tympanic membrane, or eardrum (Figure 8.25). The pinna serves to “collect” the sound waves and channel them through the external auditory canal to the tympanic membrane. Vibration of the tympanic membrane occurs at the same frequency as that of the sound wave, and the membrane depresses inward a distance proportional to the pressure exerted on it. Thus, the distance moved by the tympanic membrane determines the loudness of the sound we hear.
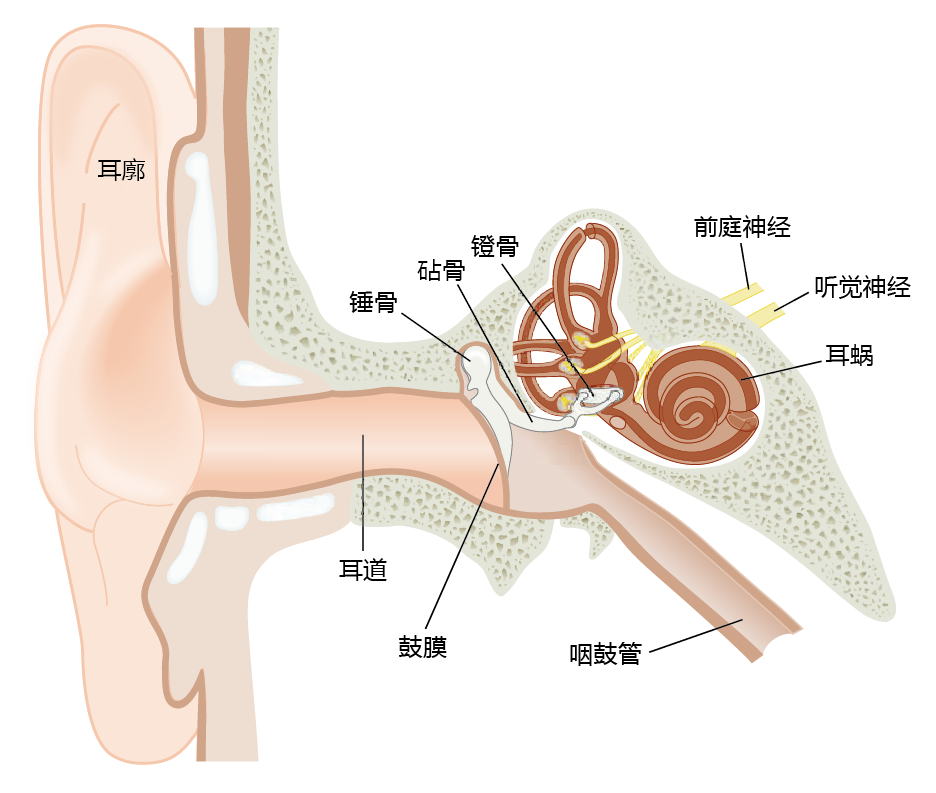
Figure 8.25 Anatomy of the human ear.
The tympanic membrane stretches across the external auditory canal and separates the outer ear from the middle ear. Vibrations of the tympanic membrane are transferred to the three bones of the middle ear, the malleus, incus, and stapes. These bones amplify the sound wave before it enters the inner ear. The inner ear contains fluids. The transmission of a sound wave through fluid is more difficult than through air, but the force per unit area on the oval window of the middle ear is considerably greater than that on the tympanic membrane, and thus the sound is amplified. The middle ear also contains the opening to the eustachian tube, which allows pressure equalization between the middle ear and the external, environmental pressure.
Conversion of a sound wave into a neural impulse occurs in the inner ear. As the stapes vibrates on the oval window, the vibrations are transmitted through the fluid-filled cochlear duct and into the cochlea (Figure 8.26). The cochlea houses the organ of Corti, which contains highly specialized receptor cells with hairlike projections called stereocilia. The stereocilia move a distance directly proportional to the vibrations of the fluid in the cochlea. As the stereocilia move, the pressure of the movement opens voltage-gated calcium channels in the receptor cell membranes. In turn, the receptor cells release neurotransmitters that stimulate the cochlear nerve, and the signal is transmitted to the hearing centers of the brain—we hear the sound.
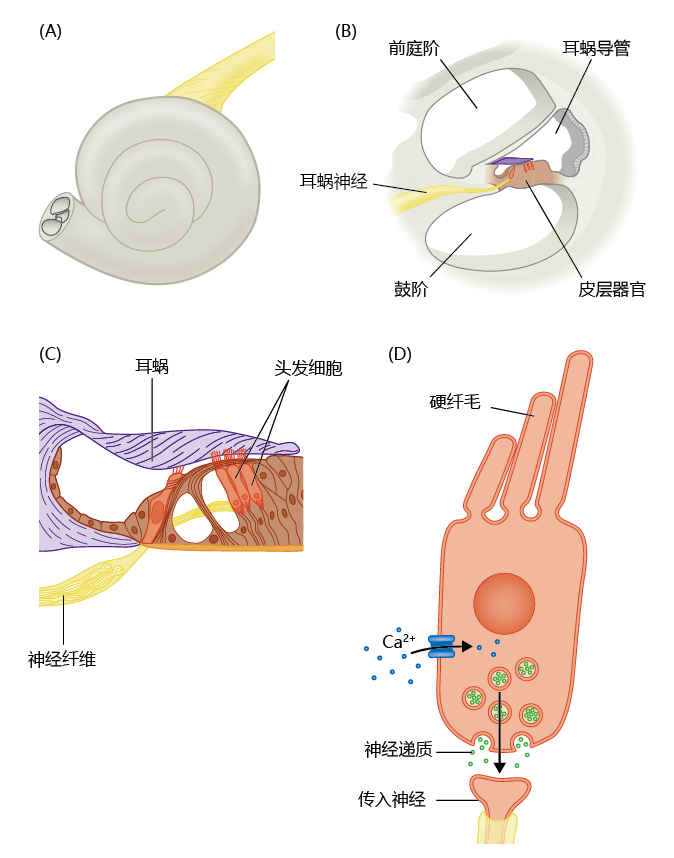
Figure 8.26 Components of the inner ear. (A) Fluid-filled cochlea. (B) The cochlea consists of three tubes that run the length of the organ. The vibration of a sound wave is transferred to the fluid of the cochlea at the oval window. These fluid vibrations change the pressure exerted against the walls of the cochlea, which, in turn, is detected by the organ of Corti in the cochlear duct. (C) Cells, called hair cells, of the organ of Corti have hairlike projections, the stereocilia, that move a distance directly proportional to the pressure exerted by the cochlear fluid. (D) Movement of the stereocilia causes voltage-regulated channels to open, and the influx of calcium into the cell induces the release of neurotransmitters—generating an electrical signal.
Loss of stereocilia contributes to time-dependent hearing loss
Most experts agree that the primary cause of the time-dependent decline in hearing, known as presbycusis, is alterations in the inner ear, although the reasons for these changes are unknown. Most notable among the many changes in the inner ear are the loss of hair cells, stereocilia, or both, in the organ of Corti. A decrease in the number of stereocilia on each cell reduces the rate of neurotransmitter release, resulting in a diminished ability to detect the loudness and higher pitches of sound. Other changes include loss of neurons throughout the auditory pathway and thickening of the capillary walls (slowing of blood flow). Presbycusis may also result from attenuation in the movement of the tympanic membrane and the bones of the inner ear. Recall that the sound wave becomes a physiological event through the transfer of sound vibrations to the tympanic membrane, causing the bones of the middle ear to move in direct proportion to the pressure. Any attenuation in the movement of these bones will cause a discrepancy between the actual wave and the sound we hear. The malleus, incus, and stapes move only as much as their ligaments and tendons allow. As we age, collagen—the primary protein of ligaments and tendons—becomes stiffer as a result of nonenzymatic cross-linking and the formation of AGEs (see Box 8.2). The cross-linking of collagen in these ligaments and tendons causes a reduction in both the distance and the speed of movement by the middle ear structures. Thus, the accumulation of AGEs may also contribute to the decline in ability to detect the loudness (amplitude) and high pitch (frequency) of sound in the postreproductive period.
Sense of sight is based on physics of light
The human eye consists of two main components that work together to create our sense of vision. The outer portion of the eye, which is exposed to the environment, contains the optics for focusing on an object and includes the pupil, cornea, iris, lens, and ciliary body (Figure 8.27). The inner portion of the eye contains the structures that transform light into a neural impulse and includes the retina, fovea centralis, optic disc, and optic nerve.

Figure 8.27 Anatomy of the human eye.
Light reflecting off objects travels in straight lines in all directions. The ability to capture light waves and develop an image of the object requires an optical system that can focus the many rays of reflected light onto a single point. The optical system of the human eye—the cornea and lens—focuses the light from external sources onto a single point on the retina, which transforms the light wave into electrical impulses for interpretation by the brain. To focus the light onto a single point in the area of the retina's fovea centralis, the cornea must bend (refract) the light rays. The amount of bending of light is referred to as refractive power; the greater the bending, the greater is the refractive power.
The human lens is attached to a sphincter-shaped (circular) muscle, the ciliary body, by zonular fibers. Relaxation of the ciliary body (the muscle elongates) stretches the zonular fibers, causing elongation of the lens (Figure 8.28). Contraction of the ciliary body (the muscle shortens) relaxes the zonular fibers, allowing the natural elastic elements of the lens to recoil and form a more spherical shape. The ability of the lens to change shape allows us to view both near and far objects. When viewing objects that are far away, the lens has a flat shape caused by relaxation of the ciliary body, and the cornea provides all the refraction. Light coming from near objects strikes the cornea at greater angles and requires a greater power of refraction. Contraction of the ciliary body causes the lens to become more spherical, increasing the refraction of light. Thus, the lens assists the cornea with refraction, and this process of reshaping the lens is known as accommodation.
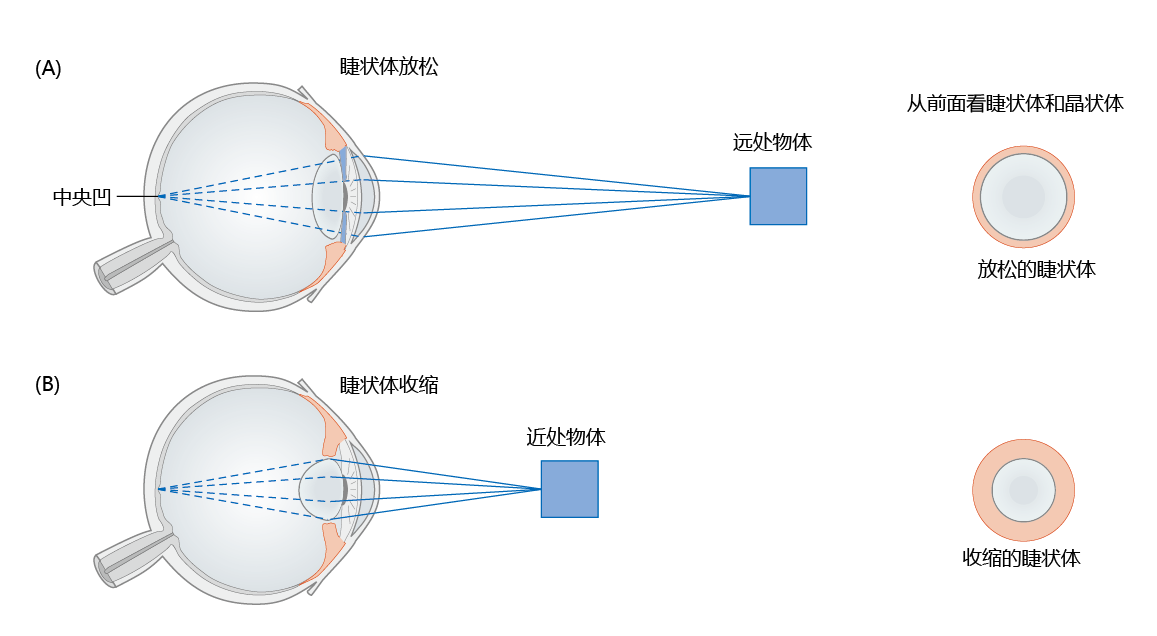
Figure 8.28 Mechanism of refraction and accommodation. Focusing by the human eye requires that light rays converge on a single point on the retina, the fovea centralis. Light reflected off far objects (A) hits the cornea at lesser angles than light reflected off close objects (B) and requires less refraction—the lens does not assist, or accommodate, the cornea and is flat in shape. Note that the lens becomes more spherical when focusing on close objects. Close objects require significant accommodation by the lens for proper refraction.
Presbyopia can be explained by time-dependent changes in refractive power of lens
All individuals over the age of 50 years have undergone changes in the optic portion of their eyes that affect their ability to focus on close objects—a condition known as presbyopia. People over the age of 40 or 50 can often be seen holding reading material at a distance from the eyes in order to focus on the print. This behavior directly reflects the inability of the lens to recoil, form a spherical shape, and increase its refractive power sufficiently to focus on near objects. Although the precise cause of presbyopia has yet to be clearly established, several factors are known to contribute. First, the cells of the lens are not replaced once they are formed; that is, they are terminally differentiated. Terminal differentiation also results in the loss of organelles in the lens cells. The consequent inability to replace or repair damaged cells in the lens can result in loss of elasticity. Second, as previously described, collagen, the protein that holds the cells together and provides the lens with its elasticity, becomes stiffer with age, and this prevents the lens from shortening into the spherical shape needed to focus on close objects. Finally, there is a slight decrease in the number of smooth muscle cells in the ciliary body; this decreases the strength of contraction and thus decreases refraction.
Terminal differentiation of lens cells leads to formation of cataracts
Approximately 3.9% of individuals over the age of 60 have visual impairments that cause low vision or blindness (Figure 8.29). Cataracts, the most common visual disease affecting the elderly, are at the interface between normal aging and disease. That is, while the clarity of the lens declines with aging, the formation of a cataract occurs in only 3.5% of individuals over the age of 60 years.
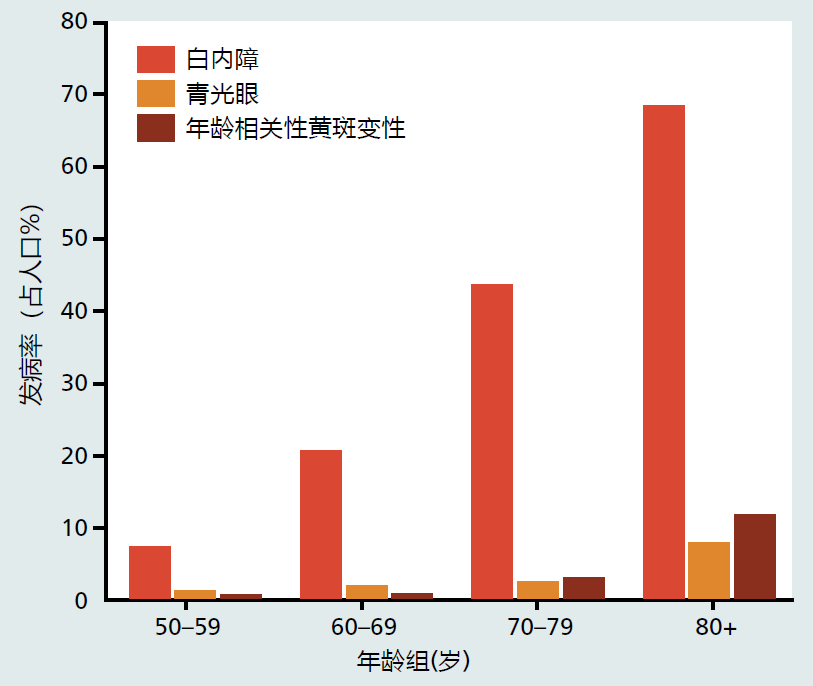
Figure 8.29 The prevalence of some time-dependent diseases of vision. (Adapted from the National Eye Institute, National Institutes of Health. 2017. Statistics and Data; https://nei.nih.gov/eyedata)
Cataracts can be defined as any opacity in the lens of the eye (Figure 8.30). The cause of time-dependent cataracts remains to be discovered, but we know that years of environmental insults such as photooxidation, increased osmotic pressure, and other stresses contribute significantly to the development of opacity. In mitotic tissues, insults to cells are prevented or repaired by processes in the cytoplasmic organelles; severe insult triggers autophagy and apoptosis leading to cell death. Because lens cells do not have cytoplasmic organelles, they cannot clean up cellular damage or trigger apoptosis. Environmental damage to the aging lens cannot be repaired.
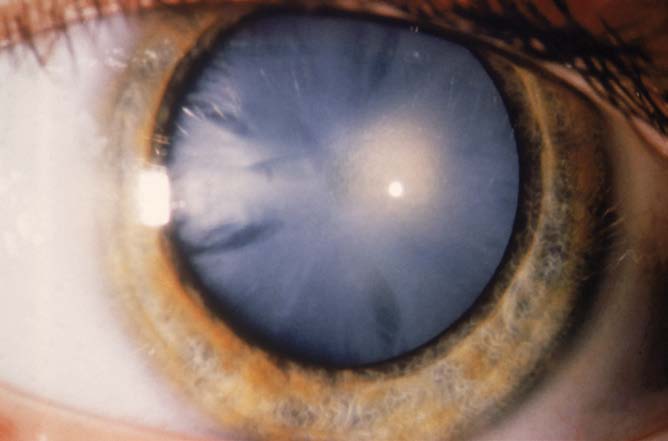
Figure 8.30 Opacity of the lens caused by the development of an age-related cataract. (Courtesy of Biophoto Associates/PR Science/Visual Photos.)
How does environmental damage to the lens lead to time-dependent cataracts? There is, as yet, no definitive answer to this question, but recent evidence suggests that protein misfolding and the development of insoluble protein aggregates provide one explanation for the time-dependent increase in lens opacity. The human lens contains considerable amounts of the protein crystallin. The tertiary structure of crystallin supports the transparency of the lens. Like all proteins, crystallin undergoes denaturation (unfolding) from time to time. Other tissues repair the unfolding of proteins with the help of chaperone proteins. The human lens does not have the apparatus to synthesize chaperone proteins. Rather, it contains α-crystallin, a protein with a chaperone-like domain. In the lenses of young humans, α-crystallin helps itself to refold and regain its function, keeping the lens transparent. The amount of α-crystallin, the chaperone domain, or both, decreases significantly with age. Denatured crystallin proteins are then unable to refold into their functional tertiary structure, and transparency is reduced.
This leads to a further question: How does the time-dependent increase in misfolded (or denatured) proteins lead to cataracts? Again, the underlying mechanism remains unknown. We do know, however, that unfolded proteins are highly susceptible to forming insoluble aggregates, and chemical analysis of cataracts reveals the presence of protein aggregates. When proteins denature, they expose amino acids that are not normally on the surface of the protein. These amino acids can then bond with amino acids in another denatured protein molecule—bonding that would not occur if a chaperone protein were present. That is, two proteins bond together, and there is no mechanism for breaking them apart. After this process occurs many times over, protein aggregate forms. (Protein aggregate formation is described in greater detail in Chapter 9.)
Cataracts can seriously impair vision, but they can be surgically removed. There are two types of cataract surgery: phacoemulsification and extracapsular cataract extraction. Phacoemulsification, the most common procedure, involves emulsifying (making soluble) the lens with ultrasonic waves. Once the lens becomes soluble, it can be removed by suction.
Phacoemulsification does not remove the lens capsule. Extracapsular cataract extraction involves removing the lens while the elastic capsule that covers the lens is left partially intact. Leaving the elastic capsule allows for implantation of an artificial lens. In both surgeries, an artificial lens is placed into the cornea. The artificial lens cannot contract or lengthen and does not correct for the loss of accommodation, although some artificial lenses that will improve accommodation are now being developed. Cataract surgery has become extremely common in the United States. Of the 3.5% of individuals over the age of 60 years who develop time-dependent cataracts, 95% have the cataracts removed.
Senses of taste and smell change only slightly with age
The mouth and nose provide us with our hedonic sense of taste, a vital sensory process that urges us to eat when we are hungry. (If you do not think this is true, think about how unpleasant or uninteresting eating becomes when you have a head cold.) The sense of taste arises from our two chemical senses, taste and smell. The sensory organs for taste, the taste buds, are found primarily on the tongue but are also located on the roof of the mouth. Flavor detection by the taste buds has been divided into five general categories: salty, sweet, bitter, sour, and umami (the taste associated with salts of glutamic acid and other amino acids). Taste buds respond to food and send signals to the brain about the type of taste by detecting changes in ionic concentration for salty and sour tastes or through the stimulation of receptors specific for sweet, bitter, and umami flavors.
Approximately 80% of the flavor we experience in food arises from our sense of smell. The olfactory nerve in the epithelium of the upper nasal cavity detects aromatic compounds in food, based on their chemical structure (Figure 8.31). Humans have more than 1,000 different olfactory receptors that are specific to groups of odoriferous molecules. Each olfactory neuron contains one to four receptors. When a scent molecule binds to the receptor, a neural signal is sent to the olfactory bulb. The olfactory bulb decodes the signal, identifying which receptor was stimulated and how much stimulation occurred, then forwards that message via the olfactory nerve (first cranial nerve) to the olfactory center of the brain (Figure 8.32). The signals from the olfactory center are relayed to the brain's limbic system, which determines whether the smell is pleasant or noxious. Areas in the limbic system also integrate the signal for taste with that for smell to give us the overall sense of taste.
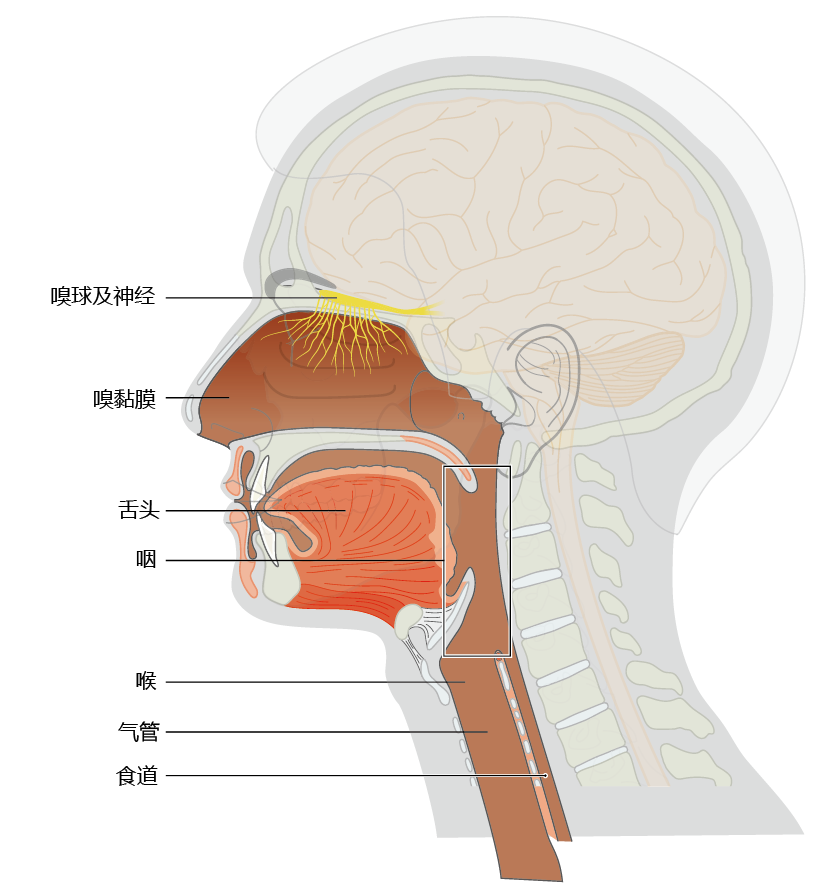
Figure 8.31 Anatomical location of the structures of the olfactory system and pharynx.
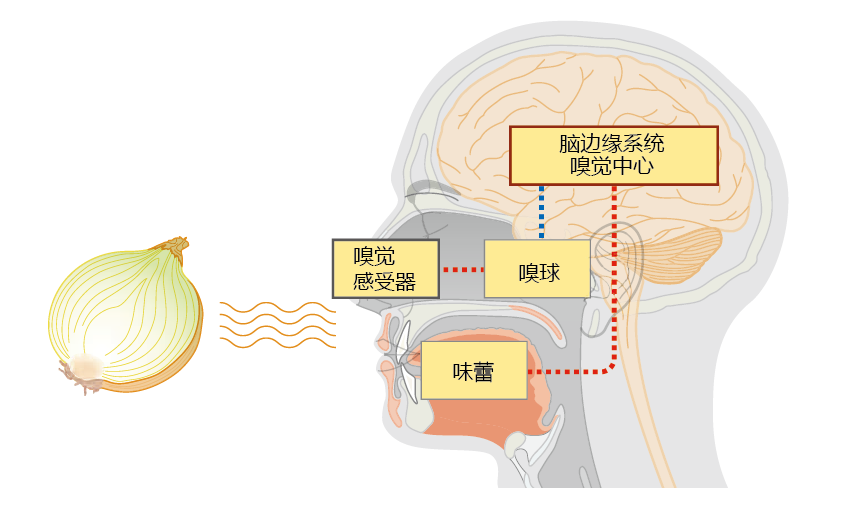
Figure 8.32 The relationship between flavor and smell. The human sense of taste depends on both the olfactory (smell) and the taste-bud systems, with the sense of smell contributing about 80% to our overall sense of taste. When food is present, the aromatic compounds attach to one of more than 1,000 specific receptors on olfactory neural cells in the nose. Binding of a chemical causes a signal to be sent to the olfactory bulb, which interprets the neural impulse. That message is then sent to the olfactory center in the limbic system of the brain. At the same time, the taste buds are stimulated by the chemical components of the food. The signals from the taste buds are relayed to the brain and integrated with the signals from the olfactory centers, and we perceive taste.
Early investigations evaluating the effect of aging on sense of taste often included individuals with diseases that affected the physiology of both taste buds and olfactory structures. This led to a general belief that the sense of taste declines with age. However, more recent and properly designed studies have, by and large, shown that time-dependent changes in taste buds and olfactory centers are minimal. To date, research has been unable to detect significant time-dependent changes in the number or neural activity of taste buds or olfactory bulbs or in the turnover of olfactory neurons. Some research suggests that the number of molecules needed to stimulate an olfactory receptor, known as the “threshold,” increases with age. But the change in threshold appears to be small—it may be associated with specific disorders and may not be physiologically significant. Some individuals have a decreased ability to discriminate between odors, suggesting a disruption in the neural circuitry that connects the olfactory receptor to the olfactory centers in the brain. These effects are not usually seen in the general population and are more likely to be associated with a disease process than with age, per se.


