8.5 CHANGES IN DIGESTIVE SYSTEM
The human digestive system, or gastrointestinal (GI) system, runs as one long, continuous tube from mouth to anus (Figure 8.33). The sole purpose of the digestive system is to extract the energy and nutrients from food that are needed for sustaining life and to eliminate solid substances that cannot be absorbed. All multicellular organisms have a digestive tract, with a basic structure that does not differ much from ours. The difference between the digestive tract of a worm and a human lies in the additional organs and systems—liver, pancreas, immune system, and neural control—that support the digestive process in humans. These additional organs allow us to eat a greater variety of foods than other species. Indeed, it could be argued that the ability to eat a wide variety of foods is one of the reasons that the human species has been so successful. We are the only species that can survive and even thrive in all parts of the world. The Inuit of the Arctic region survive and flourish on a diet consisting primarily of fish and marine mammals, as does the largely vegetarian population in the interior of Southeast Asia.
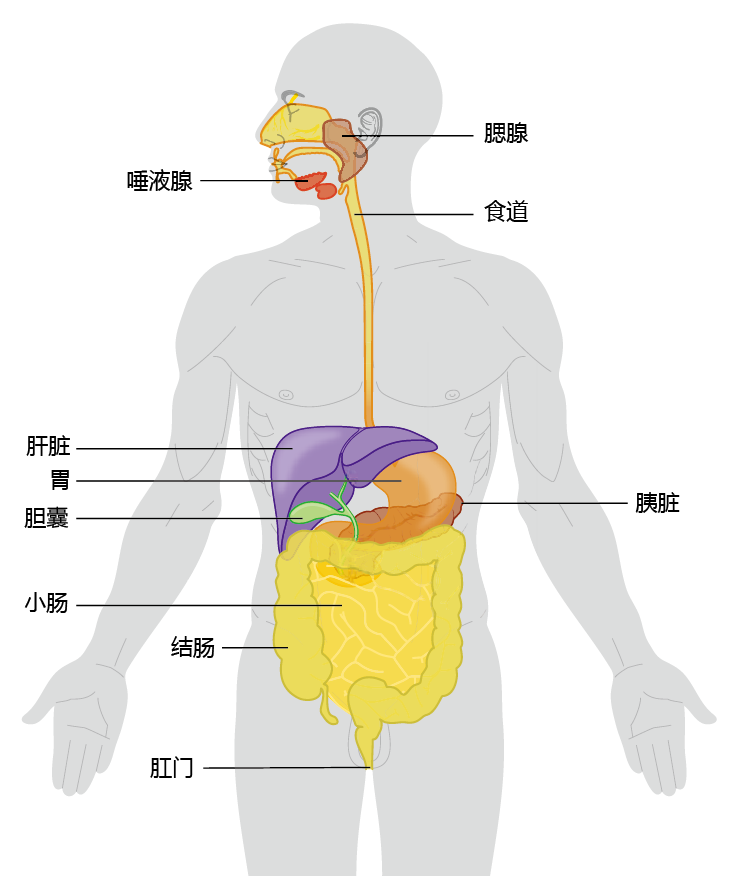
Figure 8.33 Major organs of the human gastrointestinal tract.
In general, the digestive system as a whole shows little functional decline with age. Most of the time-dependent changes are associated either with disease processes, such as cancer or diabetes, or with nutritional “bad habits.” This is not to say that aging does not affect the function of the digestive system. Common ailments such as gastritis, diarrhea, and constipation occur much more often in the elderly than in younger populations. Nonetheless, it remains unclear whether the time-dependent decrease in GI function is the result of physical changes in the system or the accumulated effects of years of poor eating habits. In this section, we examine some of the time-dependent changes that do occur, starting with the mouth and working through the small intestine. Time-dependent changes in the large intestine, rectum, and anus are associated with disease, not with aging, per se.
Time-dependent changes in mouth and esophagus do not impair digestion
Digestion begins in the mouth through the action of chewing and the initial preparation of food by the salivary glands. Chewing breaks down food into smaller pieces for ease of swallowing and creates a greater surface area for the action of the enzymes of the digestive system. The loss of teeth that can occur with age has the potential to significantly affect proper digestion, and it may limit the types of foods eaten and thus may lead to malnutrition. The reasons for time-dependent tooth loss remain controversial. While anatomical changes such as time-dependent bone loss (in the jaw) and loss in strength of the ligaments holding the teeth in position do occur, proper dental hygiene throughout life may offset these potential problems. Modern dentistry has eliminated the majority of time-dependent problems associated with poor dentition; thus, time-dependent loss of teeth does not significantly affect the digestive process for most people living in developed countries. Lack of access to dental care and lack of education regarding dental health are more likely causes of poor dentition in the elderly population than is biological aging.
The salivary glands perform several functions that prepare food for digestion by the stomach and intestines. Saliva, the product of the salivary glands, is composed of water, electrolytes, mucus, antibacterial compounds, and various enzymes. The saliva adds lubrication to food so that it can pass through the esophagus with relative ease. The water in saliva also helps to make dry food more soluble so that the aromatic compounds can be released (important for our sense of taste). Finally, saliva contains two very important enzymes, lysozyme and α-amylase. Lysozyme kills many types of bacteria and helps to prevent bacterial buildup in the mouth. Starch digestion is initiated by α-amylase by conversion of the long chains of glucose into maltose (a two-glucose molecule). Although there may be a slight decrease in saliva volume with age, the concentrations of lysozyme and α-amylase in the saliva do not change, and the decreased volume does not impair digestion. However, some neurological disorders such as stroke, Parkinson's disease, and Alzheimer's disease can alter saliva volume to the point where digestion is impaired.
Once food has been chewed and saliva added, the tongue begins the swallowing process by lifting up and pushing the bolus of food into the pharynx. The pharynx connects the mouth to the esophagus. It contains the voice box, which separates the trachea from the esophagus. The presence of food in the pharynx causes the esophagus to begin its peristaltic contractions, the rhythmic contractions of smooth muscle that push food into the stomach. Time-dependent physical disorders of the tongue and esophagus that cause problems of swallowing are relatively uncommon (as they are in all age groups). Disruption of proper swallowing most often occurs in association with psychological disorders and, like salivary dysfunction, with neurological disease.
Decline in stomach function is most often associated with atrophic gastritis
The human stomach provides four basic functions in the digestive process: (1) acts as a storage area that allows us to eat large meals that are then released slowly into the small intestine, (2) liquefies food before it enters the intestine, (3) continues the digestive process through the secretion of enzymes and other molecules, and (4) secretes exocrine hormones that prepare other organs of the digestive tract for incoming food.
The stomach lies between the esophagus and the small intestine and is divided into four regions, each having a different function: (1) the contents of the esophagus empty into the cardia; (2) the fundus, formed by the organ's curvature, adds digestive juices to the food; (3) in the body or central region, food is mixed with the digestive juices; and (4) the pylorus facilitates further mixing of the stomach contents and passage into the small intestine (Figure 8.34). The cells of the stomach wall secrete hydrochloric acid (HCl) and enzymes necessary for the digestive process (Figure 8.35). The mucosa layer contains the glands that secrete HCl and other digestive enzymes; the submucosa, a matrix of connective tissue, provides structure for the blood vessels; muscles in the muscularis layer contract the stomach for mixing of the food; and the connective tissue of the serosa forms the barrier between the stomach and other organs of the digestive tract. The cells of the stomach wall are arranged into glands that form gastric pits, where food comes in contact with the HCl and digestive enzymes. Each region of the stomach has a slightly different arrangement of glands and different cells within the glands (TABLE 8.3).
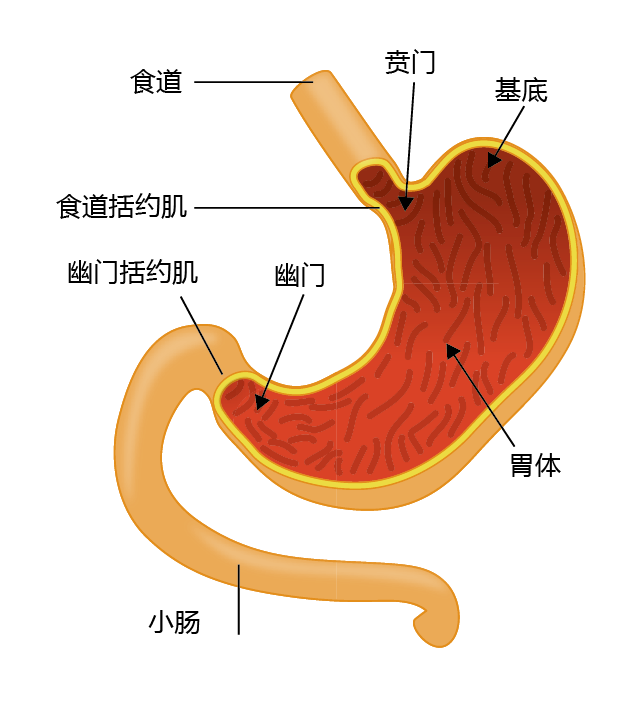
Figure 8.34 Anatomy of the human stomach.
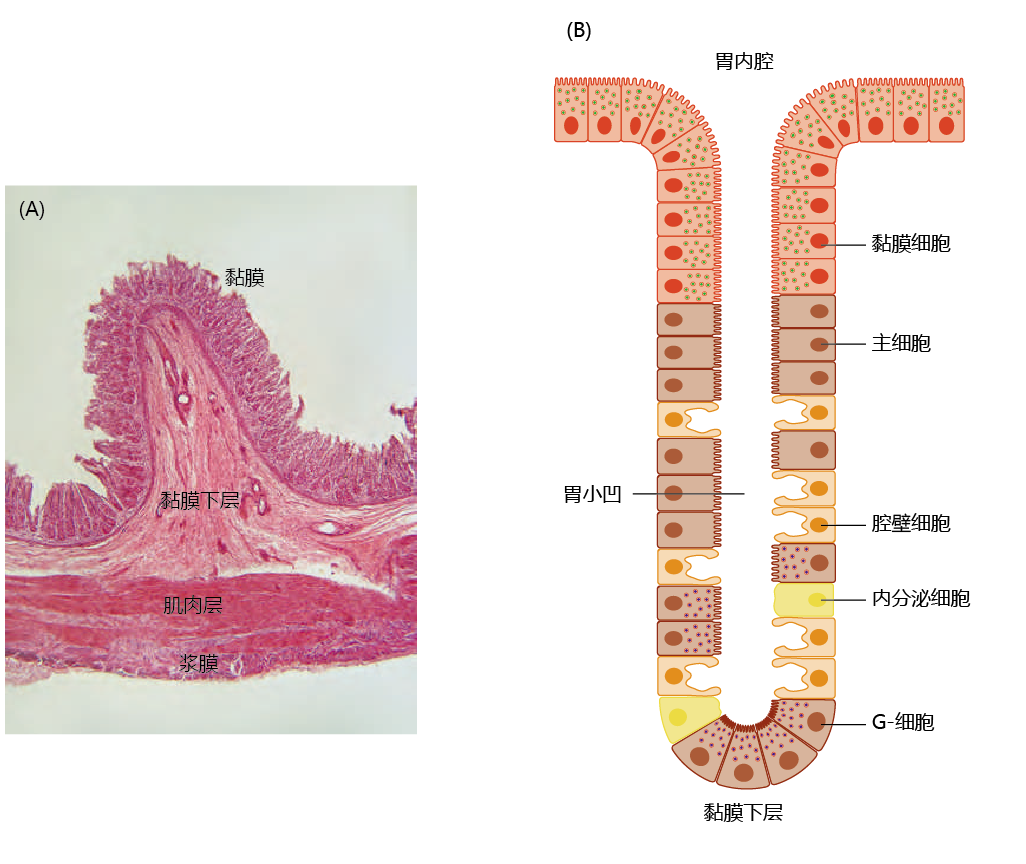
Figure 8.35 The layers of the stomach wall. (A) Cross-sectional micrograph of the stomach wall in the region of the fundus, showing its four layers. (B) Arrangement of cells in the glands of the mucosa layer that form the gastric pits. Glands in different parts of the stomach may contain different proportions of the four types of stomach cells (see Table 8.2), but the anatomical arrangement is the same. Mucosa cells, which secrete protective alkaline mucus, are at the top, followed by chief cells and parietal cells, with G-cells at the bottom of the pit. (A, courtesy of J. Harshaw/Shutterstock.)
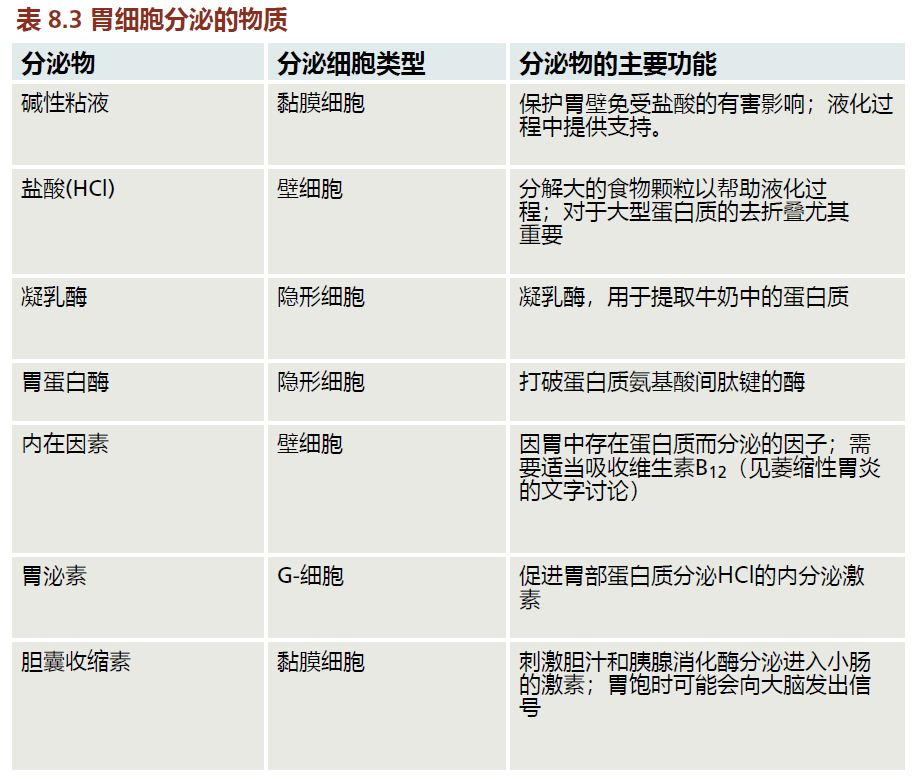
When food enters the stomach, the parietal cells secrete HCl. Hydrochloric acid helps to break down large particles of food into smaller pieces, which are then liquefied into a semisolid fluid called chyme before entering the intestine. In addition, the acidic nature of the stomach causes the unfolding of large proteins (denaturation). This allows the enzyme pepsin, secreted by the stomach, to more efficiently break the bonds between amino acids (only peptides of two or three amino acids and single amino acids can be absorbed by the intestinal cells). The secretion of HCl also stimulates the secretion of other enzymes and hormones needed for proper digestion.
Evaluating possible time-dependent anatomical or physiological decline in the stomach can be challenging, due in large part to a condition known as atrophic gastritis. Atrophic gastritis causes inflammation of the stomach mucosa, leading to reduced numbers of parietal and chief cells (Table 8.3). These cells are replaced by fibrous tissue. As a result, the stomach's secretion of essential substances, such as HCl, pepsin, and intrinsic factor, is impaired. Atrophic gastritis can lead to severe digestive problems and nutrient deficiencies. For example, decreased secretion of intrinsic factor by parietal cells can lead to vitamin B 12 deficiency and the development of megaloblastic anemia (the production of underdeveloped red blood cells). While there are many causes of atrophic gastritis, about 90% of cases are the result of persistent infection by the bacterium Helicobacter pylori (H. pylori). When treated with the appropriate antibiotic, atrophic gastritis can be cured and the problems associated with the disorder alleviated. The question remains, however, why the aged population has a greater incidence of H. pylori infection. The answer awaits further research.
Changes in small intestine can affect digestion and nutrient absorption
The digestion of food and absorption of nutrients occur primarily in the small intestine in what can best be described as a mass-action process. The chyme—the mixture of water, food, and digestive juices—moves through the intestinal lumen by peristaltic contraction. The peristaltic action also causes mixing of the chyme so that enzymes aiding in digestion come into closer contact with the nutrient molecules. When chyme enters the small intestine from the stomach, its presence stimulates the release of hormones that induce the pancreas to secrete several digestive enzymes and the gallbladder to release bile salts (Figure 8.36). Fat digestion begins when the bile salts, secreted into the intestine from the liver via the gallbladder, emulsify lipids and thus increase their solubility. The greater surface area created by the bile salts increases the efficiency of the lipases secreted from the pancreas, enzymes that break apart large fat molecules into free fatty acids. Pancreatic enzymes catalyze reactions that break the bonds holding together the sugars in carbohydrates, the amino acids in proteins, and the fatty acids in lipids.
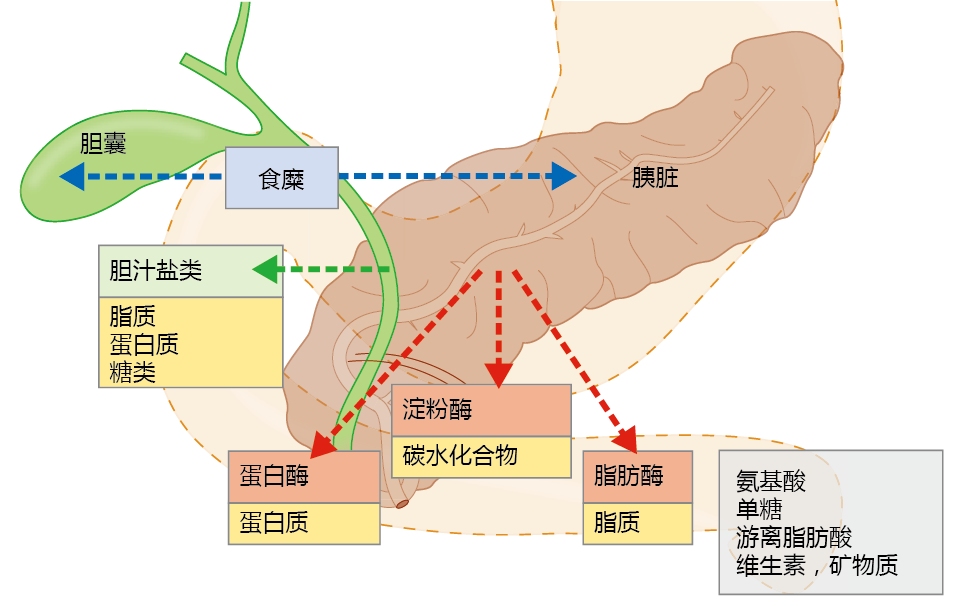
Figure 8.36 Digestion in the small intestine. As the chyme enters the small intestine from the stomach, it stimulates cells (blue arrows) at the interface between the stomach and small intestine to release hormones that, in turn, stimulate the release of bile salts from the gallbladder (green arrow) and digestive enzymes from the pancreas (red arrows). Bile salts increase the solubility of fats, and digestive enzymes break apart large molecules of lipids, proteins, and carbohydrates into their smallest possible components: free fatty acids, amino acids, and monosaccharides, respectively. Vitamins and minerals are normally bound to these large molecules and are released during the digestive process.
The intestinal wall has structures called villi that increase the surface area of the intestine and enhance absorption (Figure 8.37). The epithelial cells also have protrusions on their membranes called microvilli, which form the brush border; this further increases surface area.
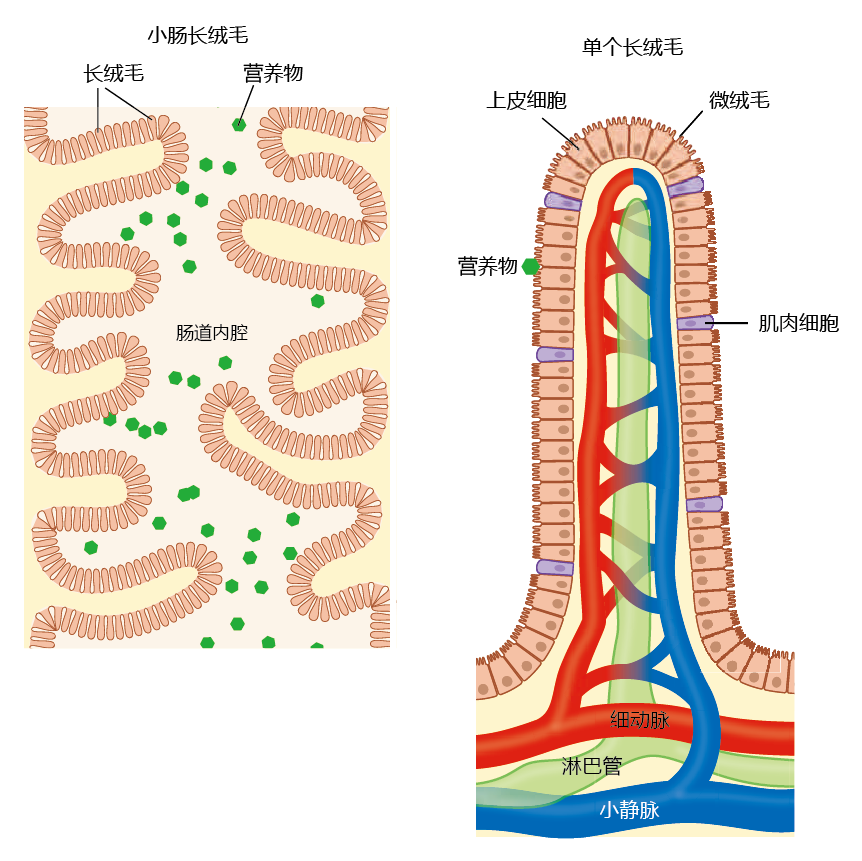
Figure 8.37 Macroanatomy and microanatomy of the small intestine. The interior surface of the small intestine (left) contains protrusions into the lumen that are covered with villi. The villi increase the surface area of the small intestine and thus enhance absorption. The surface of each villus (right) is covered with epithelial cells that also contain protrusions that increase surface area: the microvilli, forming the brush border. Absorbed nutrients pass through the epithelial cells and into the general circulation via capillaries in the villi.
The human digestive process has evolved to break down food into its smallest basic nutrient components—fatty acids, monosaccharides and disaccharides, amino acids, vitamins, and minerals—so as to facilitate their absorption. Three types of absorption are used by the small intestine: passive diffusion, protein channels, and mediated transport. Recall from Chapter 4 that the cell membrane (plasma membrane) consists primarily of nonpolar (without a net charge) lipids. Small nonpolar molecules in the intestinal chyme—free fatty acids, some vitamins, and a few amino acids—are highly soluble in the nonpolar membrane and are absorbed by passive diffusion. That is, these molecules diffuse through the membrane into the intestinal cell without the help of membrane proteins (Figure 8.38).
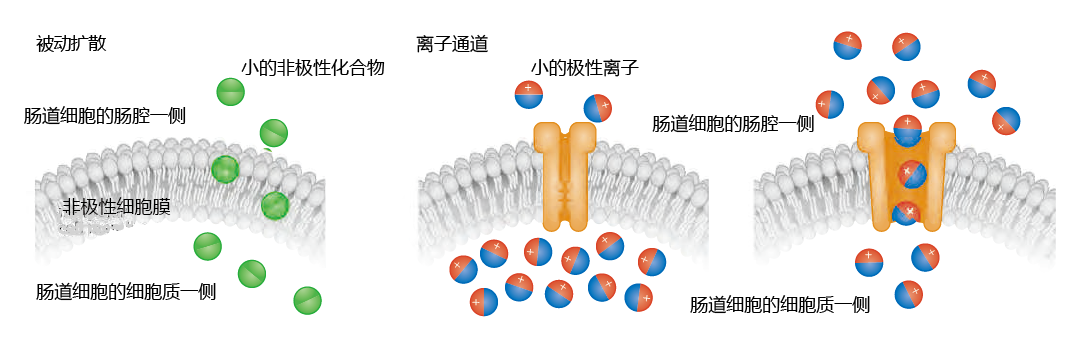
Figure 8.38 Passive diffusion and ion channels. Small nonpolar molecules can pass through the plasma membrane of the intestinal epithelial cell unobstructed, because the membrane is also nonpolar. Small polar ions are absorbed into the intestinal cell through ion channels. The channels open only when the concentration of the ion in the chyme (in the intestinal lumen) is greater than the intracellular concentration.
Ions are small and highly polar (having a net negative or positive charge) and cannot be absorbed by passive diffusion. Rather, ions are absorbed through channels in the membrane (Figure 8.38). The permeability of ion channels depends on a concentration gradient between the contents of the chyme in the intestinal lumen and the cytosol of the intestinal cell. When the concentration of the ion is higher in the chyme than in the intestinal cell, the channels open and ions flow through the membrane. When the intracellular ion concentration exceeds that in the chyme, the channels close.
Mediated transport makes use of specialized membrane proteins to transport large polar and nonpolar molecules across the membrane. There are two types: facilitated diffusion and active transport. Facilitated diffusion occurs when the concentration of the molecule is greater in the chyme than in the intestinal cell. This is a simple process by which the molecule binds to a specific transport protein in the cell membrane that facilitates transport into the cell. The monosaccharide fructose is used as an example of facilitated diffusion in Figure 8.39A. Active transport also uses membrane proteins to transport large molecules across the membrane, but this is a two-step process (Figure 8.39B). In the first step, the molecule to be transported, with a lower concentration in the chyme than in the intestinal cell, binds to a transport protein in the cell membrane. Because of the higher concentration inside the cell, the molecule cannot overcome the unfavorable concentration gradient without help. That help usually comes in the form of a small ion with a favorable concentration gradient: higher in the chyme than in the cell. By using the ease of transport associated with the ion, the large molecule enters the cell against its concentration gradient. However, the increase in intracellular concentration of the ion would eventually inhibit further transport of the ion and, with it, the large molecule, if the ion is not removed from the cell. The second step of active transport uses energy to move the ion from the cell back into the intestinal lumen, against its concentration gradient.
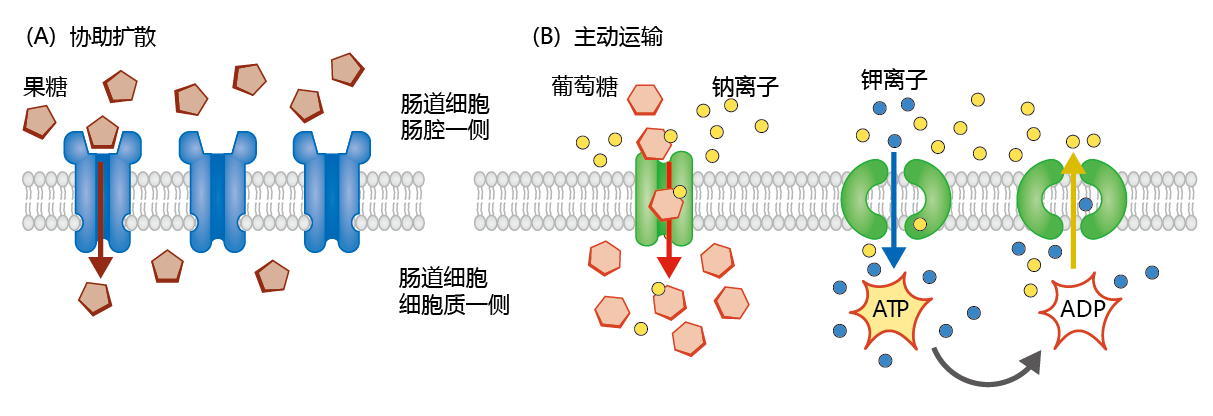
Figure 8.39 Facilitated diffusion and active transport. (A) Facilitated diffusion occurs when large polar or nonpolar molecules, such as fructose, attach to a specific transporter molecule and are transported into the cell. Facilitated diffusion predominates when the concentration of the molecule is higher outside (in the chyme) than inside the intestinal epithelial cell. (B) Active transport is a two-step process that uses a co- transporter. For example, glucose is bound to its transport protein along with sodium. The favorable concentration gradient of sodium (lower inside the cell) helps glucose enter the cell against its concentration gradient. Sodium is then “pumped” out of the cell using the energy of ATP and the exchange of potassium ions for sodium. This pump, called the sodium/potassium ATP pump, helps to maintain the electrical potential across the plasma membrane in many types of cells.
The effects of aging on the form and function of the small intestine are somewhat controversial, because we do not know the full extent of the influence of disease and poor eating habits on normal function. Many early studies on the effect of aging on intestinal function included subjects with various diseases, and tissue samples were often collected during surgery. Not surprisingly, these initial studies suggested that age has a detrimental effect on the small intestine, including the malabsorption of nutrients. However, with the introduction of safe and somewhat pain-free biopsy methods, tissue samples have been collected from the small intestine of healthy individuals. These investigations show that villi number and height (a measure of surface area) do not change significantly with age. Moreover, the replicative capacity of epithelial cells, a property critical to maintaining absorption in the small intestine, does not change with advancing age.
Proper digestion and absorption rely on the ability of the intestinal tract to move the chyme efficiently through the lumen by peristaltic action. Attenuation of this rhythmic muscular action can lead to a decrease in flow and the development of constipation—a condition often associated with age. There does seem to be a time-dependent reduction in the force of contraction generated by the intestinal smooth muscle, resulting in a slight increase in transit time of the chyme. However, this slight increase does not result in increased constipation or malabsorption syndromes. Time-dependent constipation more likely reflects poor eating habits than physiological changes in the small intestine.
Controversy also exists concerning the effect of age on the absorption of nutrients. Traditionally, the absorption of micronutrients—essential vitamins and minerals needed in only very small quantities for proper growth and metabolism—has been suggested to decrease with age. This view has recently been challenged by the finding that specific proteins that transport micronutrients do not decrease with age. Nonetheless, calcium absorption may decline with age in women and may be associated with a decrease in the activity of vitamin D, the vitamin needed for proper absorption of calcium. The reasons for a time-dependent decrease in vitamin D activity are unclear, but it is most likely associated with the normal postmenopausal loss in bone mineral content (see Chapter 9). The finding that calcium absorption does not seem to decline with age in men suggests that malabsorption of this mineral reflects normal hormonal shifts during the postreproductive period in women. That is, the time-dependent decline in calcium absorption may be closely associated with gender rather than a universal effect of aging.


