9.7 SKELETAL SYSTEM AND BONE CALCIUM METABOLISM
Human bone has three major functions: (1) it provides strength and structure to the human form; (2) it is the site of blood cell production; and (3) it stores calcium and is involved in the regulation of serum calcium concentration. Calcium is the most abundant mineral in the body. It interacts with phosphorus to form calcium phosphate, the hard, dense material that forms our bones and teeth. In fact, the human skeleton contains 99% of the body's calcium. The rest of the calcium is in the form of serum calcium ions (Ca2+). Serum calcium is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation and many enzymatic processes. In this section, we discuss the importance of serum calcium and calcium regulation; in the next section, we examine time-dependent bone loss.
Parathyroid and thyroid hormones balance blood calcium
The concentration of serum calcium needs to be maintained within a very narrow range of about 9–10 mg/dL. The maintenance of this Ca2+ concentration is so important that evolution has provided a gland, the parathyroid gland, with the sole function of regulating serum Ca2+ (Figure 9.38). If the concentration of Ca2+ drops below 9 mg/dL, the parathyroid gland secretes parathyroid hormone (PTH). This hormone facilitates increased serum Ca2+ by increasing the release of calcium from bone, increasing intestinal Ca2+ absorption, and increasing reabsorption of Ca2+ by the kidney tubule. If Ca2+ levels rise above 11–12 mg/dL, the thyroid gland secretes the hormone calcitonin. Calcitonin has the opposite effect of PTH: it facilitates inhibition of calcium release from bone. Calcitonin has little to no effect on Ca2+ absorption by the intestine and reabsorption by the kidney tubule. Serum Ca2+ levels of 10–11 mg/dL do not stimulate calcitonin secretion. Rather, serum Ca2+ concentrations in this range are reduced to normal by increased urinary excretion.
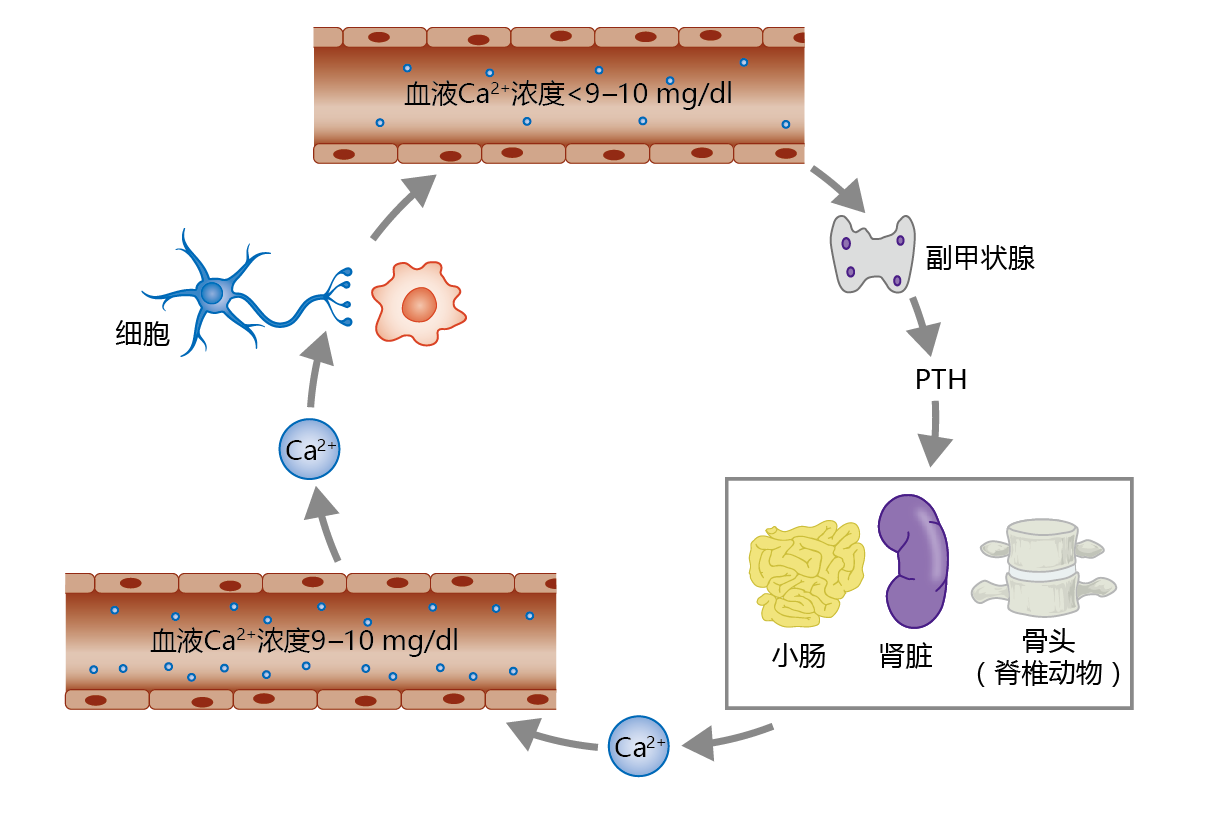
Figure 9.38 Regulation of serum calcium (Ca2+) by parathyroid hormone (PTH). The parathyroid gland, located just above and behind the thyroid gland in the upper neck, releases PTH in response to blood Ca2+ concentrations below 9 mg/dL. PTH stimulates increases in Ca2+ reabsorption by the kidney tubule, Ca2+ resorption from bone, and Ca2+absorption by the small intestine. Serum Ca2+ is extracted from the blood by cells and used for several different physiological functions, including maintenance of neural conduction, muscle contraction, and intracellular signaling.
Hormones regulate balance between bone mineral deposition and resorption
Hormones also help regulate the process of bone remodeling, the continual breakdown and renewal of adult bone tissue (Figure 9.39). There are two types of bone: trabecular (or spongy) and compact. Trabecular bone is highly vascularized, and its structure creates a large surface area for bone-vessel contact. That is, Ca2+ can move into and out of bone more easily in trabecular bone than in compact bone. The bone cells responsible for remodeling include the osteoblasts, osteoclasts, and, to a lesser degree, the osteocytes. In response to PTH, osteoclasts secrete proteolytic enzymes that break down collagenous matrix containing the Ca2+ salt hydroxyapatite (Ca10[PO4]6[OH]2). Hydroxyapatite gives bone its hardness and strength. Osteoclasts also secrete acids that break apart the hydroxyapatite molecule into a variety of Ca2+ -linked amorphous salts, primarily calcium phosphate Ca3(PO4)2. Formation of these amorphous salts serves two critical functions. First, the salts provide a pool of Ca2+ that can be released quickly in response to PTH. Second, the salts are used by osteoblasts in the formation of new bone tissue.
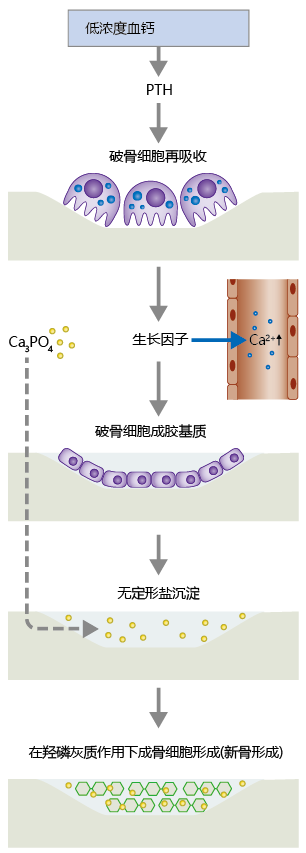
Figure 9.39 The process of bone remodeling. When serum Ca2+ concentration falls below 9 mg/dL, the parathyroid gland secretes parathyroid hormone (PTH), which activates osteoclast activity. Osteoclasts digest the bone matrix, releasing amorphous salts, growth factors, and Ca2+ ions. The growth factors stimulate conversion of preosteoblasts to mature osteoblasts, which fill in the resorbed area with collagenous matrix. Osteoblasts also release alkaline phosphatase, which aids in the precipitation of amorphous salts in the newly formed collagenous matrix. Over time, the amorphous salts form hydroxyapatite, which leads to the production of osteocytes and new bone.
The resorption of bone Ca2+ by osteoclast activity leaves a hole in the bone that was previously occupied by hydroxyapatite crystals. Without the formation of new bone tissue, bone mineral content would decrease and the bone's overall strength would diminish. New bone formation begins when macrophages release growth factors during their “cleanup” of the products of osteoclast activity. These growth factors—including transforming growth factor beta (TGF-ß), platelet-derived growth factor (PDGF), and insulin-like growth factors 1 and 2 (IGF-1 and IGF-2)—stimulate differentiation of the preosteoblast into the mature osteoblast. The mature osteoblast secretes matrix material, primarily collagen, into the demineralized area of the bone. It also secretes alkaline phosphatase, an enzyme that aids in precipitation of amorphous salts into the newly formed matrix. Over the next 2–3 months, stochastic processes transform amorphous Ca3PO4 salts into hydroxyapatite crystals, ending the remodeling cycle for this section of bone.
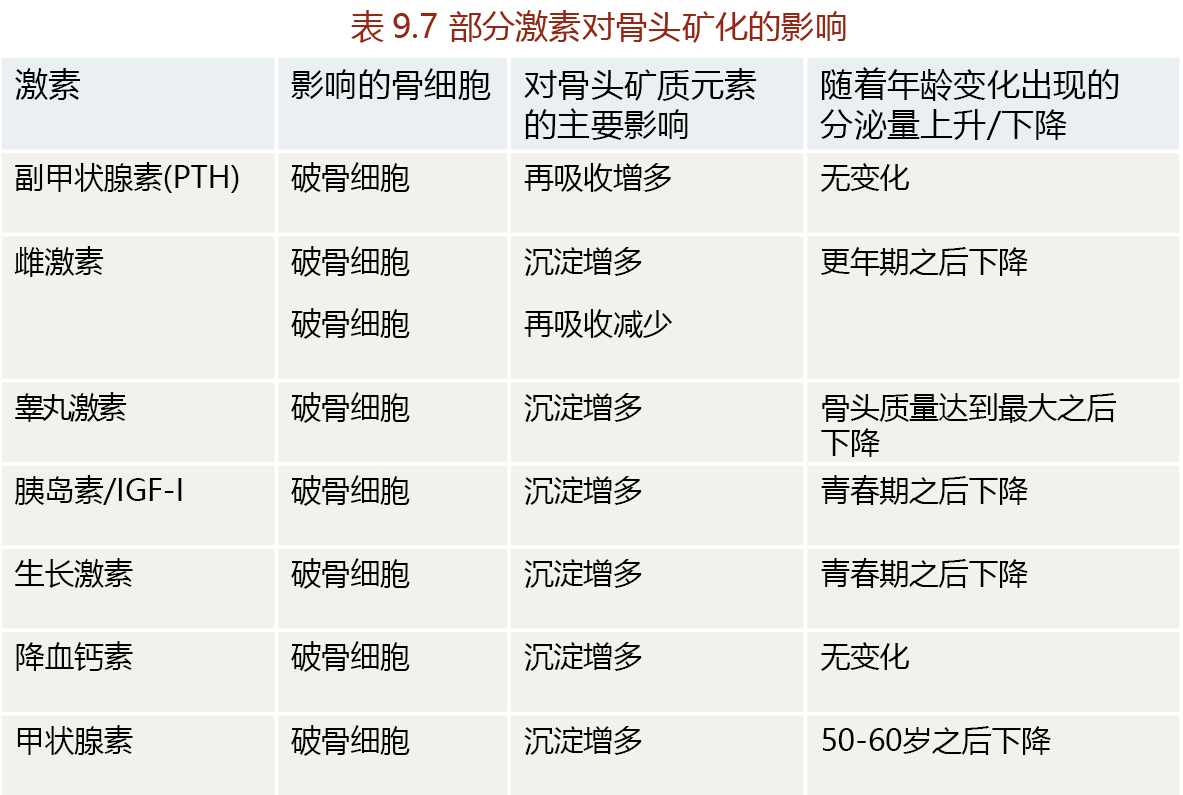
Bone remodeling occurs continuously throughout the life span and is regulated by several hormones and growth factors (TABLE 9.8). Greater bone mineralization during early development (pre-puberty) reflects the stimulatory influence on the osteoblast of growth-inducing hormones and growth factors such as growth hormone, insulin, IGF-1, and calcitriol (vitamin D3). Testosterone and estrogen in both men and women promote bone growth at puberty. Testosterone seems to stimulate the differentiation of preosteoblasts into mature osteoblasts. Women with high levels of testosterone have a longer period of bone growth after the start of puberty and thus have greater peak bone mass. Estrogens promote bone growth in two ways: (1) by stimulating osteoblast activity and (2) by inhibiting osteoclast activity. While the exact mechanism by which estrogen inhibits osteoclast activity remains unknown, this hormone may act by stimulating the osteoblast's release of a protein (osteoprotegerin) that inhibits the secretion of proteolytic enzymes by osteoclasts.