9.8 TIME-DEPENDENT DISEASES OF BONE: OSTEOPOROSIS
The average height of women and men decreases after the age of 50 years. The loss in height reflects a slight compression of the vertebrae caused by declining bone mineral content after peak bone mass has been achieved (Figures 9.40 and 9.41). The accelerated loss of bone mass in women after menopause (∼50+) reflects declining estrogen levels. Declining estrogen levels place women at a significantly greater risk of developing osteoporosis, a bone disease characterized by low bone mass and strength, which leads to an increased risk of fracture. In fact, 80% of all cases of osteoporosis occur in women. Although men also experience time-dependent bone loss, prevalence of osteoporosis is significantly less than that observed in women. The lower prevalence rate seen in men reflects the much slower decline in bone mineral content after the age of 50 (Figure 9.40). Younger men can, however, develop secondary osteoporosis, caused by such things as medication, cancer, and kidney disease.
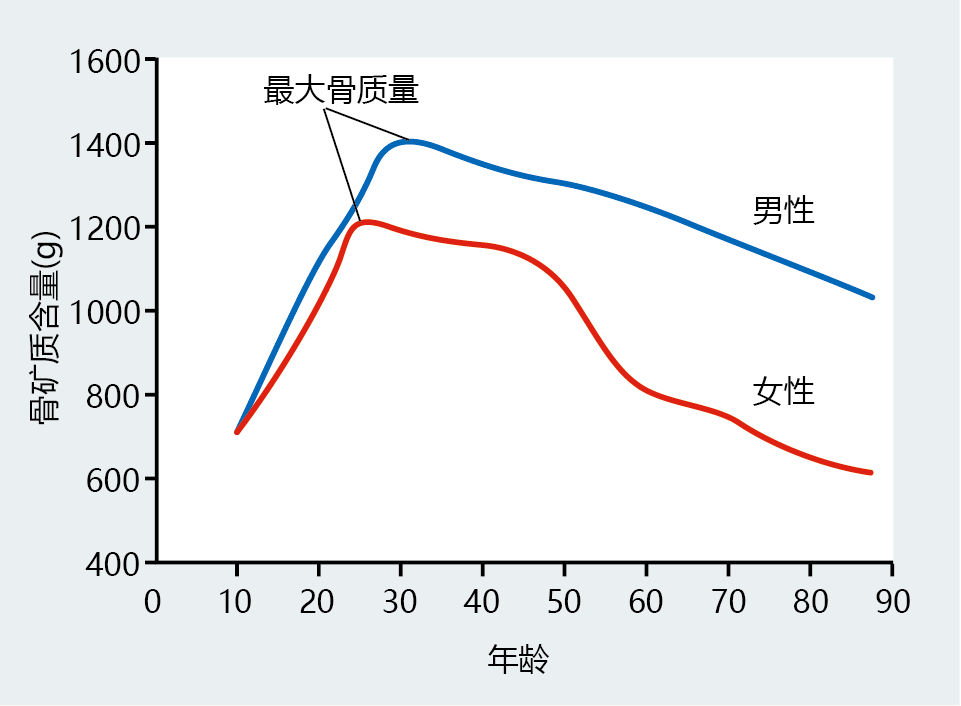
Figure 9.40 Total body bone mineral content (BMC) in males and females throughout the life span. Females tend to achieve peak bone mass at a younger age than males. The slow rate of BMC loss after peak bone mass is approximately the same for men and women, until menopause (50+ years of age). Note the accelerated rate of decline in BMC in women after the age of 50. (Data from World Health Organization. 2003. Prevention and management of osteoporosis. WHO Technical Report Series 921. Geneva: World Health Organization.)
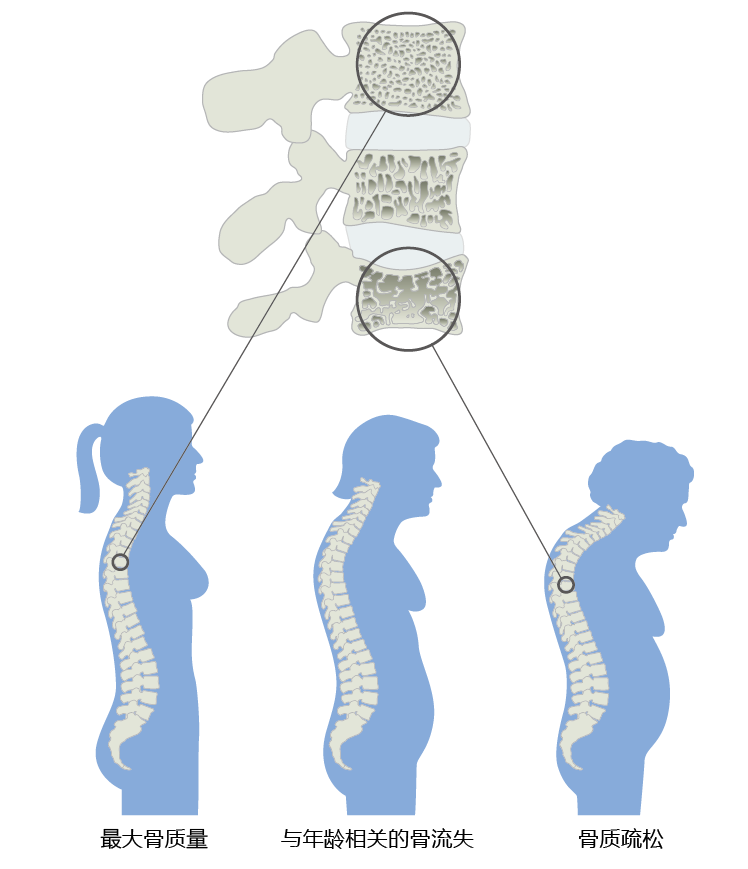
Figure 9.41 Progression of vertebral bone loss in women, causing a decrease in height. The age-related loss of bone calcium results in compression of the vertebral body, which causes the entire spinal column to shrink, decreasing height. Excess bone loss in the vertebrae results in osteoporosis and a curvature of the spine sometimes known as a dowager's hump.
The increased rate of bone loss in women after menopause provides us with a rare opportunity to explore, in humans, the relationship between time-dependent loss and reproductive life span. In this section, we discuss the development of primary osteoporosis in women from a perspective of reproductive senescence, exploring how normal bone mineral loss transitions into osteoporosis in postmenopausal women.
Increased rate of bone mineral loss at menopause can lead to osteoporosis
The increased rate at which women lose bone mineral after menopause places them at a significant risk for developing osteoporosis. The World Health Organization (WHO) has suggested a diagnostic classification of osteoporosis as a bone mineral density (BMD) (measured in grams of mineral/cm 2) at the hip or spine of 2.5 standard deviations (SDs) below the young population's normal mean (Figure 9.42). For ease of comparison across age groups, the WHO refers to the statistical distribution (mean ± SD) of the young normal population as a T-score. T-scores of ≥ −1 are considered normal and pose no additional risk for developing osteoporosis. Values between −1 and −2.5 indicate a low bone mass, clinically known as osteopenia, and an increased risk of developing osteoporosis. Interestingly, the normal BMD for women over the age of 60 declines to the low bone mass range, suggesting that age is a major risk factor for osteoporosis. Although the WHO distribution indicates that women 90 years of age and older normally have osteoporosis, limited data for this population make these values unreliable.
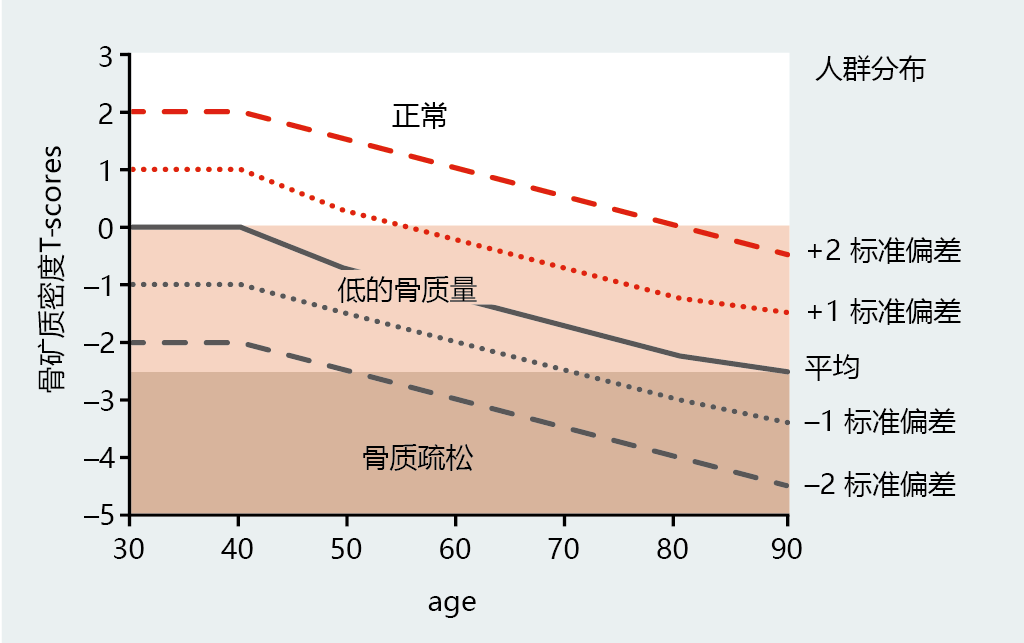
Figure 9.42 The WHO's diagnostic criteria for osteoporosis in women. Osteoporosis is defined as a bone mineral density (BMD, g/cm 2) at the hip or spine of ≤ 2.5 standard deviations below the young normal mean reference (premenopausal, 30–40 years of age). This value is presented as a T-score (left y-axis). The graph lines represent the population distribution of BMD for women at various ages (right y-axis). (Data from World Health Organization. 2003. Prevention and management of osteoporosis. WHO Technical Report Series 921, Geneva: World Health Organization.)
Environmental factors influence risk of developing osteoporosis
More than 100 years ago, Julius Wolff, a German surgeon, found that bones become thicker—that is, BMD increases—when placed under increased load. His observation led to the development of Wolff's law: bones grow in direct proportion to the load placed on them. For example, the BMD of the radius (the large bone in the forearm) in the dominant arm of professional tennis players is consistently greater than that of the radius in the nondominant arm. Intensive load-bearing exercise increases BMD, and disuse of bone leads to a decrease in BMD. This effect has classically been observed in patients undergoing long periods of bed rest and in astronauts after spending time free from Earth's gravitational force. Thus, lack of physical activity can increase the risk of developing osteoporosis. However, the effectiveness of moderate exercise programs in increasing BMD remains uncertain. The greatest gains in BMD among postmenopausal women who start a light to moderate weight-bearing exercise program occur in previously sedentary individuals. Only small gains in BMD are observed in women who exercise regularly.
Recall that estrogen modifies the full effect of PTH-induced bone resorption by stimulating osteoblast deposition of bone mineral and inhibiting resorption by osteoclast. When estrogen concentrations decline during and after menopause, the bone dumps greater amounts of Ca2+ into the serum, and, in turn, excretion of calcium in the urine increases in order to maintain serum Ca2+ in the 9–10 mg/dL range. The increase in urinary calcium can often be greater than dietary intake, a phenomenon known as negative Ca2+ balance. Increasing the dietary intake of Ca2+ often eliminates a negative Ca2+ balance in young, premenopausal women and helps to maintain bone mass. However, similar treatment in postmenopausal women has not been as effective in maintaining premenopausal levels of BMD. The National Academy of Sciences has set the recommendation for daily Ca2+ intake by postmenopausal women at 1200 mg/day. This amount has proved effective in combination with medication and increased physical activity for previously sedentary individuals.
Intestinal absorption of dietary Ca2+ requires vitamin D. Older individuals' declining dietary intake and limited exposure to ultraviolet light (skin synthesizes vitamin D on exposure to UV light) may decrease the amount of vitamin D available for appropriate Ca2+ absorption. Increasing the dietary intake of vitamin D has been shown to increase the absorption of Ca2+ in postmenopausal women. However, the vitamin D–induced increase in Ca2+ absorption has not been shown to increase BMD in nonosteoporotic, postmenopausal women. Nonetheless, because the data are conflicting, clinicians often recommend increasing the intake of vitamin D along with Ca2+ as a precautionary measure.
Women's bone mineral content reaches its peak at the age of ∼25 years. Thereafter, bone mineral content slowly declines until menopause, when the rate of mineral loss sharply increases. The rate of loss in both the pre- and postmenopausal age groups does not depend on the amount of bone mineral at the time of peak bone mass. This phenomenon is shown nicely in Figure 9.39. Women with a peak bone mineral content ≥1 SD above the mean have the same rate of loss as do other groups. However, these women do not reach the osteoporosis threshold during their life span. In fact, women with exceptionally dense bones (+2 SD above mean) may not even reach the low bone mass threshold. In other words, the more bone mineral you have at peak bone mass, the more bone mineral you will have later in life. Attaining one's genetic limit for bone mineral content at the time of peak bone mass has been widely accepted as a primary prevention strategy for reducing one's risk of developing osteoporosis.
Recommendations aimed at helping young women achieve their maximal peak bone mass have, for the most part, focused on improving dietary Ca2+ and vitamin D intake, as well as increasing weight-bearing exercise. The chances of achieving maximal peak bone mass are greatly enhanced if young women follow the recommendations suggested by the Food and Nutrition Board of the National Academy of Sciences. That is, girls under the age of 18 years should consume at least 1300 mg of Ca2+ and 5 mg of vitamin D per day. In addition, high dietary intake of specific vitamins and minerals should be accompanied by increased weight-bearing physical activity.
Drug therapies can slow bone loss in postmenopausal women
Focusing on preventive measures for young women does not imply that older women will not benefit from exercise or dietary improvement. However, increasing weight-bearing activity, dietary Ca2+, and vitamin D levels only slows, and does not prevent, bone loss in pre- or postmenopausal women. Stopping the loss and increasing bone mineral content after peak bone mass or menopause can be achieved only through pharmacotherapy. However, increased physical activity along with increased Ca2+ and vitamin D intake in combination with pharmacotherapy significantly enhances bone mass and reduces the rate of fracture compared with drugs alone. Here we look at three of the more common types of drugs used to increase bone mass in postmenopausal women: estrogen, specific estrogen receptor modulators (SERMs), and bisphosphonates.
Since estrogen stimulates osteoblast activity (bone formation) and inhibits osteoclast activity (bone resorption), it should not be surprising that estrogen replacement (also known as hormone replacement therapy [HRT]) has found significant acceptance as a therapeutic agent in preventing postmenopausal bone loss. Virtually every study investigating the use of HRT as a treatment for osteoporosis has found increased bone mass, decreased rate of fracture, or both. Unfortunately, recent evidence reveals that the use of HRT at levels needed to increase bone mass also increases the risk of breast cancer. The increased risk of breast cancer has significantly reduced the use of estrogen as the first line of defense against excessive bone loss and osteoporosis. Estrogen replacement for prevention or treatment of osteoporosis is now relegated to use when other treatments fail or when the risk of health problems due to fracture outweighs the risk of cancer.
SERMs are a class of compounds that have selective actions, depending on which estrogen receptor, α or ß, they bind to. For example, tamoxifen (Nolvadex) increases bone density by stimulating osteoblast activity but inhibits the growth of breast cancer tissue by blocking the binding of endogenous estrogen to its receptor. Tamoxifen's severe side effects and relatively weak action on bone have led to its use primarily as a cancer treatment. Raloxifene (Evista) was developed specifically to treat osteoporosis, although it has recently been approved for treating women at high risk for breast cancer. Clinical trials have found that raloxifene is more effective than tamoxifen at reducing hip and vertebral fractures but is less effective than estrogen. Raloxifene does not produce the severe side effects observed with tamoxifen—namely, uterine inflammatory diseases and an increased risk of uterine cancer.
Bisphosphonates are a group of compounds that induce apoptosis in osteoclasts and thus inhibit bone resorption. Bisphosphonates disrupt normal ATP synthesis by creating a carbon-phosphate bond in place of the normal oxygen-phosphate bond. Because ATPase can break only the oxygen-phosphate bond, the carbon-phosphate ATP builds to toxic levels in the osteoclast and induces apoptosis. The three FDA-approved bisphosphonates are alendronate (Fosamax), ibandronate (Boniva), and risedronate (Actonel). All three are equally effective at slowing the rate of bone mineral loss in postmenopausal women and decreasing the risk of hip and vertebral fractures. There remains some controversy as to whether bisphosphonates help increase bone mass or simply stop the loss of bone mineral content.


