1.3 衰老生物学家怎么研究衰老:用实验室生物研究人类衰老
伦理上,实验不允许用于人类,因此,衰老生物学家们使用其它各种生物,包括单细胞生物,非脊椎动物例如昆虫,哺乳动物和鱼类,鸟类,非人类的灵长动物以及人类中的一些遗传病来调查人类衰老的本质。本部分简短介绍真核生物(具有细胞核)作为实验模型来模拟人类衰老机制(原核细胞无细胞核,尚未确定它们在衰老研究中的地位)。最后一部分“衰老学家门如何研究衰老:比较衰老生物学”我们用野生动物模拟人类衰老和寿命。
无论所使用的生物类型如何,这些研究都将和人类衰老相关联(BOX 1.1)。系统遗传学描述了基于基因相似性的生物之间的关联性。
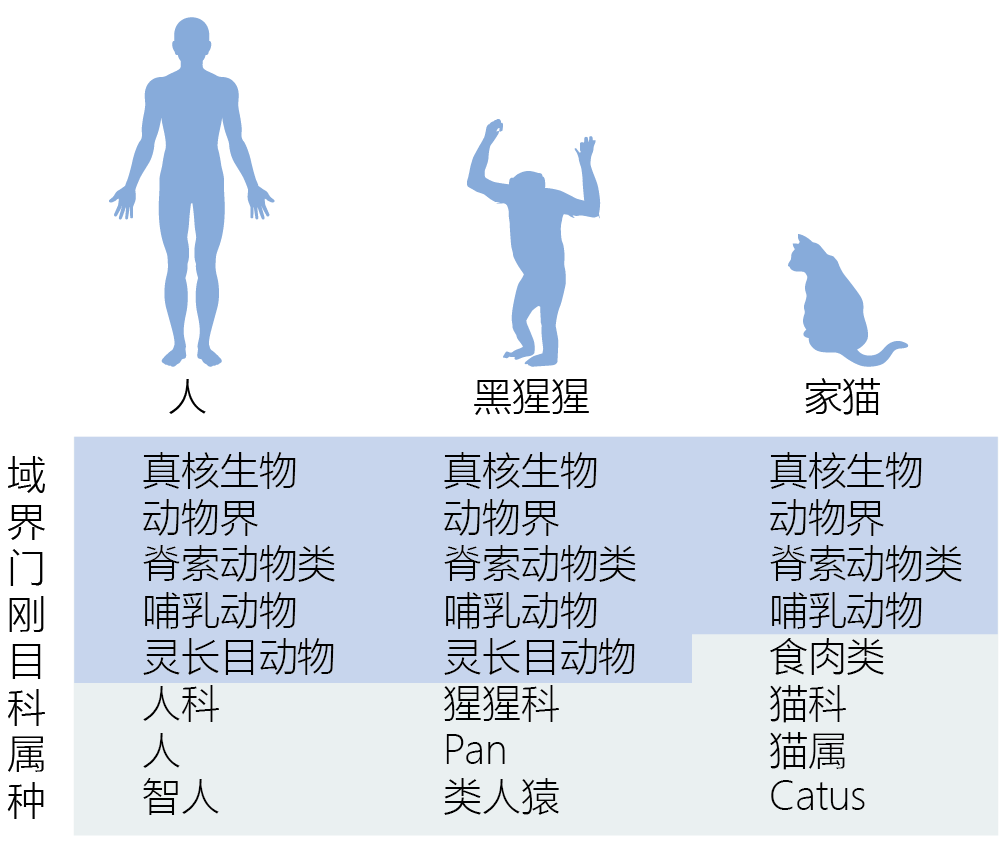
| BOX 1.1 生物系统树 |
|
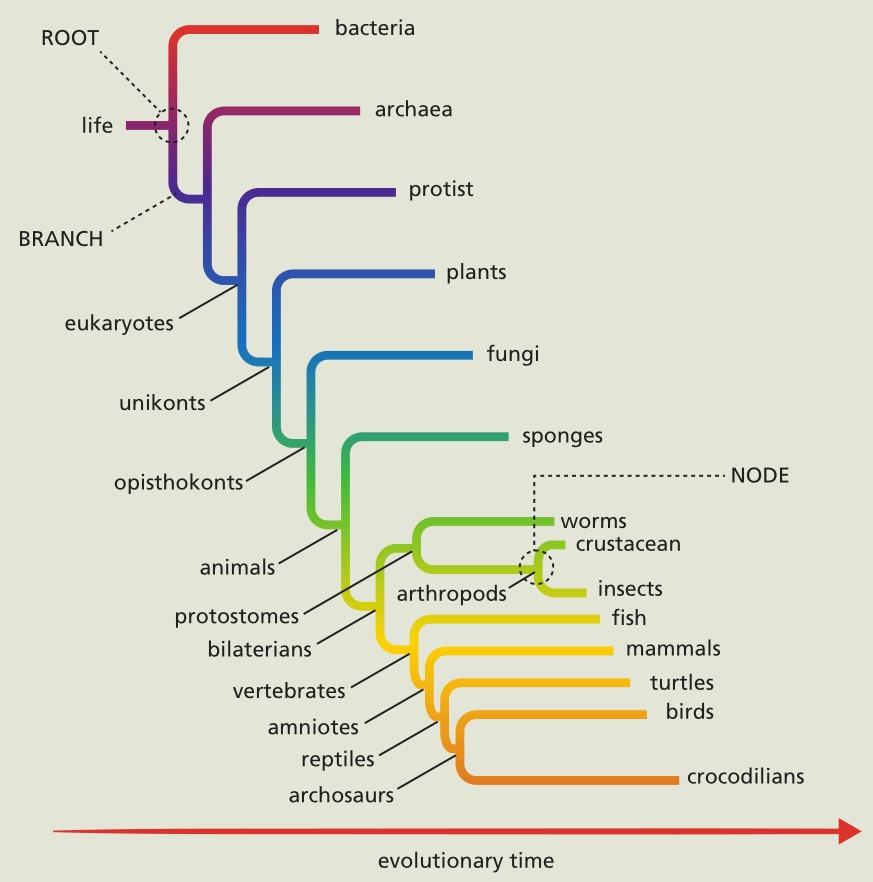
21世纪之前,生物的分类学以及它们之间的关系受到哲学和神学教育的影响。分类学的建立者—John Ray(1627–1705)和Carolus (Carl) Linnaeus(1707–1778)—开创他们的生物分类,挑战神创论。200年来,形态学(生物的形状和结构)使用林奈的系统分类学显示,进化的形式是越来越复杂的—细菌是最简单的以及最早的生命形式,人类是最复杂而且是最后进化出来的。(图1.5) 图1.5 林奈分类系统。林奈的分类系统是基于生物体和其他生物的相似性而排列的一系列登记排列的目录。虽然形态分类正在被系统遗传学分类系统所取代,但林奈开发的分类名称仍然被广泛使用。 当孟德尔遗传定律被重新发现并理解后,生物学家开始思考,生物进化真的是遵循从低等到高等的有序过程吗。随着发现DNA结构在所有生物体中都是相同的,并且在“较低等”的生物中发现的有些基因与“高等”的生物中发现的基因形同,使用另一种分类方式的趋向就变得强烈了。这些发现提供了确凿的证据,证明了所有生物都来自一个共同的祖先,或者说几个共同的祖先。此外,进化理论者发现,对于一种生物而言,无法从形态学复杂程度来证明其进化历史。物种只不过是为了在环境中生存需要而变得复杂而已。因此,复杂性与其说是人类有目的的定义,还不如说是物种为了在环境中生存的能力。 20世纪后半叶,由于生物科技的进步,分类系统的发展开始基于系统发生而不是形态学了。系统发生学指的是一种或一组生物的事件的进化顺序。现代系统发生学使用一种混合的因素和技术建立物种间的进化关系。这些因素包括形态特征;DNA序列,生态数据以及数学算法来预测可能的基因关系。系统发生学并不关心一个物种是否比另一个物种更高等。它只是表明了一个物种从它之前的形态进化是由于近似环境的遗传适应。 系统发生关系可以形象化为系统发生树,这个分支图像表明了不同物种间的进化关系(图1.6)。树的分支定义了祖先和后代关系。一个分支包含了同一个祖先的所有后代。分支节点表示分类学单位——例如一个生物,一个物种或一个种群。 . . . . The topology, or branching pattern, of the tree can be scaled or unscaled. A scaled tree uses 分支长度比例to the 进化改变的数量 that have occurred between 分类学单位。 An unscaled tree uses the branches only to connect 关系。 Trees may also be rooted or unrooted. A rooted tree, such as the one in 图1.6, has a 共同祖先 for all other species or groups on the tree. Unrooted trees illustrate only 关系,without reference to共同祖先。
图1.6 A rooted, unscaled phylogenetic tree. A phylogenetic tree is composed of nodes, each representing a taxonomic unit (species, populations, individuals), and branches, which define the relationship between the taxonomic units in terms of descent and ancestry. For example, arthropods are a monophyletic group that contains insects and crustaceans. In the tree, arthropods are represented by a node, and insects and crustaceans are represented by branches off that node.
Phylogenetics is more than just a system for classification, however. It is a useful tool. For example, gene sequence comparisons made by molecular phylogeneticists have established a close 进化关系 between humans and the domestic pig, indicating a close relationship in physiology. Indeed, the pig heart is very similar to the human heart in both structure and function. Medical researchers used this information to test whether healthy heart valves taken from a pig could be transplanted into a failing human heart. As it turns out, pig valves are an almost perfect match for human valves. Today, many people are alive and well with heart valves from pigs that replaced their own defective valves—due, in part, to the science of phylogenetics. |
The discussion here serves as an introduction to groups of species generally used in laboratory experimentation in the science of biogerontology. Later chapters explore in much greater detail the specific use of these model systems. Plants are not discussed here; Chapter 6 describes plant biogerontology in detail. It is important to remember that no single animal or plant model is a “perfect” system for studying 生物衰老. The choice of an organism, instead, depends on such things as the question being asked, the rate of aging and longevity of the organism, the organism‘s reproductive type and success, and the cost for the care and keeping of the organism.
分离的细胞系统可被用来研究衰老和寿命的基本生物化学
人类是地球上最复杂的生物,containing sophisticated neural, vascular, and 内分泌系统 that have allowed us to be 进化arily successful. Nonetheless, to work efficiently, these advanced systems depend on the proper functioning of their intracellular biochemistry. This is why cell function and its changes over time will ultimately describe how humans age. Investigating aging from a cellular perspective has a long history in gerontology, dating back to 1912, when the first cell culture was successfully developed (see Chapter 4). From these early studies arose the four basic cell systems that are used in the study of biogerontology: primary cell cultures, replicating cell cultures, cell lines, and stem cells.
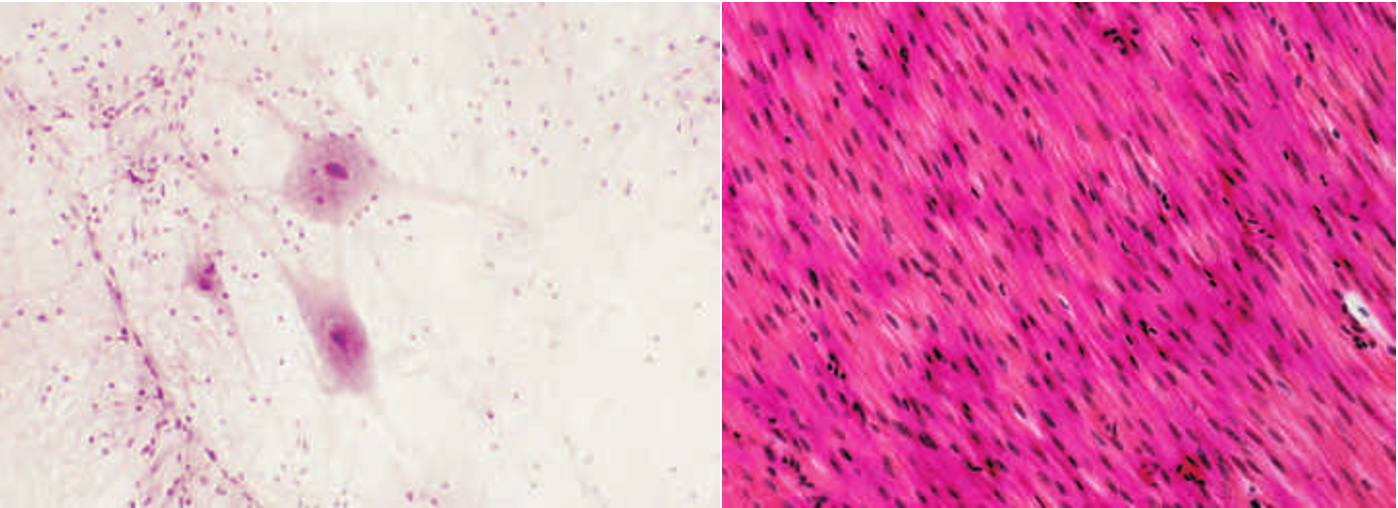
Primary cell cultures are differentiated cells (highly specialized cells) removed directly from their in vivo location and maintained in an in vitro 环境 (Figure 1.7) . In biogerontology work, primary cell cultures typically contain post-mitotic cells, or cells with limited proliferation capacity, and remain viable for a very short time, usually only a few days. Primary cell cultures allow researchers to compare differences among particular types of differentiated cells. For example, techniques are available for determining the contractile properties of smooth muscle cells. To this end, smooth muscle cells for young and old animals can be removed, placed in culture, and then evaluated for age-related differences.
Figure 1.7 Primary cell cultures. (A) Human neurons. (B) Smooth muscle cells. (A, courtesy of Thinkstock; B, courtesy of S. Gschmeissner/Getty Images.)
Replicating cell cultures are the most widely used type of cell culture systems in biogerontology. Replicating cell cultures are nondifferentiated mitotic cells, such as fibroblasts, that have been removed from tissue and allowed to divide until they reach confluence (maximum capacity within the confines of the culture dish). They are then separated into another flask and allowed to grow again, a process known a population doubling. Mammalian cells can be doubled about 30–50 times before the population dies. By sampling cells within the culture at different times, the biogerontologist can compare intracellular factors in young versus old cells. These systems are generally used to evaluate factors that lead to cell senescence and death, since mitotic cells have a finite replicative life span in vitro.
Cell lines are mitotic cells that do not have a finite life span. These cell populations either are derived from cancerous tumors or are normal cells that have had their internal biochemistry altered to make them immortal. Cell lines are a staple in general cell biology research, but they have not found wide use in biogerontology, most likely because these cells do not age and do not display the age-related functional loss observed in normal cells. However, some researchers use cell lines to investigate the pathways common to aging and cancer.
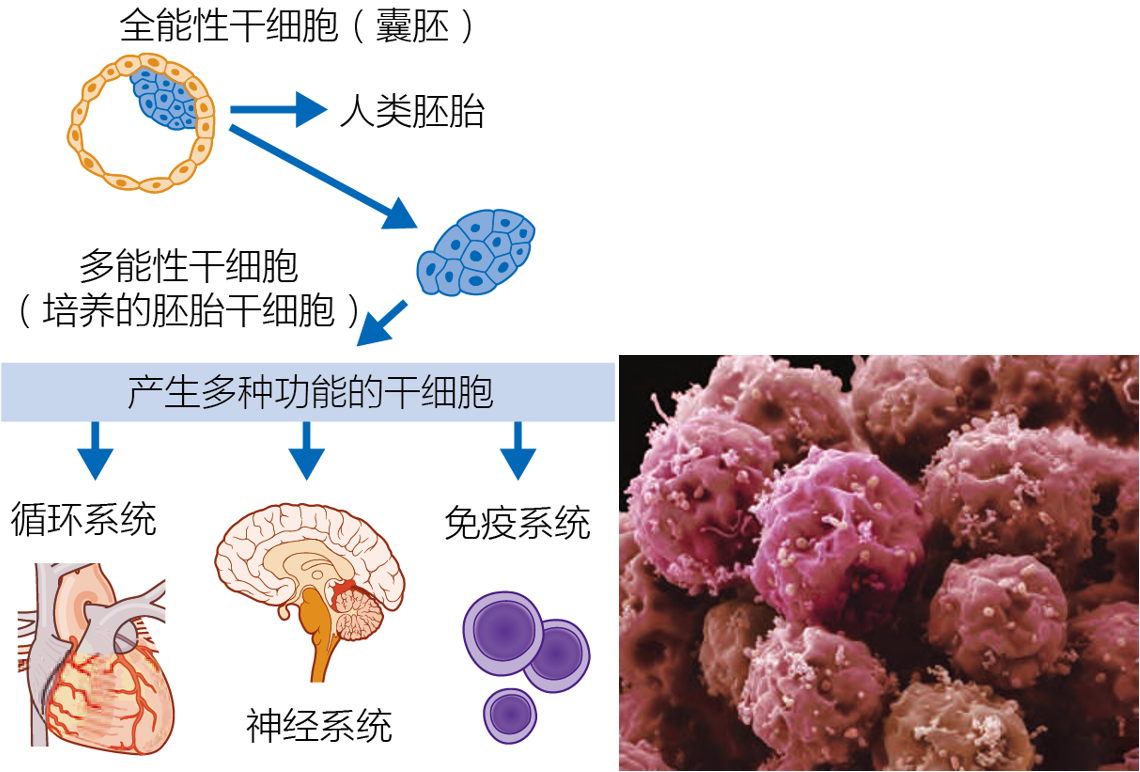
Stem cells are undifferentiated cells that have the ability to renew themselves indefinitely; they divide and create a differentiated cell. Stem cells exist in two forms, embryonic and adult. Embryonic stem cells are either totipotent, having the ability to generate an entire organism, including the placenta, or pluripotent, having the ability to generate cells and tissues from the three types of germ layers—endoderm, ectoderm, and mesoderm (Figure 1.8) . Adult stem cells are multipotent and form the type of tissue from which they were extracted: liver stem cells produce liver cells, muscle stem cells produce muscle cells, and so on. Stem cells have value in biogerontological research because of their ability to rejuvenate or replace aging tissue. For example, hematopoietic stem cells, which produce blood cells, are being implanted into the bone marrow of elderly patients following chemotherapy, to reduce the risk of infection by speeding up the regenerative process.
Figure 1.8 Embryonic stem cells can give rise to the different cell types of the body. (A) Embryonic stem (ES) cells are harvested from the inner cell mass of a blastocyst. These cells can be encouraged to differentiate into specific cell types. (B) A colony of embryonic cells in culture. Because of their unlimited capacity for selfrenewal, embryonic stem cells have been proposed as a mechanism to regenerate tissues and organs damaged by aging or age-related disease. (B, courtesy of S. Gschmeissner/Science Photo Library/Getty.)
真菌是研究影响衰老和寿命的环境因子的理想材料
Fungi, in yeast and mycelial form, do not have a sophisticated vascular, neural, or endocrine system, making intercellular signaling difficult (cell-to-cell communication occurs through gaps or pores in the cell wall). They must rely on direct cellular contact with the 环境 for sensing the world around them. This property makes fungi an excellent subject for the study of 环境al factors that affect aging. In addition, the study of aging in fungi provides researchers with some practical advantages. First, fungi survive in virtually every environment on Earth (Figure 1.9) . A fungal species can be selected to match the 环境条件 that the investigator hypothesizes has an effect on aging. Second, the nuclear and mitochondrial genomes of fungi have a compact, high coding–to–regulatory sequence ratio and are comparatively easy to sequence. As you will see in Chapter 5, the high coding–regulatory sequence ratio allows investigators to determine more precisely which gene has which function. Third, fungi have a wide range of life spans, ranging from a few days to 8000 years. And fourth, large quantities of individual fungi can be grown quickly in the laboratory at very low cost.
Figure 1.9 The diversity of fungi. Fungi live in diverse 环境s and have a wide range of life spans. (A) Budding yeast (Saccharomyces cerevisiae) is easily grown in culture. (B) Honey mushroom (Armillaria ostoyae; also called shoe-string fungus) may be the oldest living organism on Earth. A single A. ostoyae discovered in the Malheur National Forest of northeastern Oregon may be as old as 8000 years. Both (C) goblet fungus (Cookeina sulcipes), found in tropical rain forests, and (D) reindeer moss (Rangifera) which grows on the tundra, are examples of long-lived fungi that can survive in harsh conditions. (A, courtesy of S. Gschmeissner/Science Photo Library/Corbis; B, courtesy of M. Watson/moodboard/Corbis; C, courtesy of M. Read/123RF; D, courtesy of A. Romanov/123RF.)
原始无脊椎动物may provide clues to extended细胞生命,细胞信号和整体衰老(Primitive invertebrates may provide clues to extended cellular life, cell signaling, and whole-body aging)
Primitive invertebrates are a diverse group that includes sponges, jellyfish, sea anemones, coral, worms, rotifers, and mollusks (Figure 1.10) . Many aquatic invertebrates have extreme life spans and have only recently received significant attention in aging research (see the next section, “How Biogerontologists Study Aging: Comparative Biogerontology”).

Figure 1.10 Long-lived anemones. Sea anemones, such as this giant green anemone (Anthopleura sola), are reported to have extreme life spans and unlimited growth. (Courtesy of altrendo nature/Thinkstock)
Worms and rotifers are easy to raise in the laboratory, and most have relatively short life spans. Although cell and tissue specialization in these organisms is primitive compared with that of higher-order animals, these species have sophisticated intercellular communication through cell junctions. Because these animals also have a compact genome, they are excellent models for investigating how cellular events may be linked to whole-body aging. Chapter 5 provides a detailed description of how the genetic manipulation of cell-signaling pathways connecting the 环境 to the start of reproduction in C. elegans led to the discovery of genes that may regulate longevity. Moreover, these organisms are eutelic; that is, they have a fixed number of cells when they reach maturity. Because they cannot renew tissue, species within this group may provide biogerontologists with a model to investigate stochastic aging, a principle of whole-body aging that you will learn about in Chapters 3 and 4.
Insects can用于调查how whole-body and 细胞内 signaling affect life history(Insects can be used to investigate how whole-body and intracellular signaling affect life history)
Insects are the single largest class of animals on the planet, with three million known species and several times that number of undiscovered species. The short life span and extremely high rate of reproduction of many insects provide the opportunity to study and manipulate the genetics of several generations in a short time. In addition, the life history (the sum of all biological events occurring in an organism throughout its life span) of insects is more easily modulated through manipulation of the 环境 than are those of many other, more complex animals. For example, the reproductive activity of insects, and thus their life spans, can be altered through changes in temperature, food availability, and amount of light in the day. This type of modulation is often associated with changes in signals from the neuroendocrine system. Thus, researchers can use insects to investigate how whole-body and intracellular signaling affect an organism‘s life history.
Although the benefits of insects as models for human aging are clear, only a handful of species have been studied to any great extent. The fruit fly, Drosophila melanogaster, has been used extensively in aging research and was the first animal to have its life span precisely determined. D. melanogaster‘s niche in aging research lies primarily in the study of the genetics of life span and is discussed in great detail in subsequent chapters.
在调查营养,遗传和生理问题上,小白鼠是最常见的实验材料
The vast majority of biogerontological research has been conducted on rats or mice as model organisms. Rodents are particularly useful in research because of the similarity between rodent and human physiology and cellular function. Rats and mice are relatively inexpensive to house compared with other animals with similar life spans. And unlike for human subjects, the diet and 环境 of rodents can be strictly controlled. In addition, rodents can easily be genetically manipulated, which allows for the testing of gene products and age-related changes. Because so much of the current research has been conducted on these animals, their specific use in research is described in detail in later chapters.
Nonhuman primates display many of the same time-dependent changes as humans
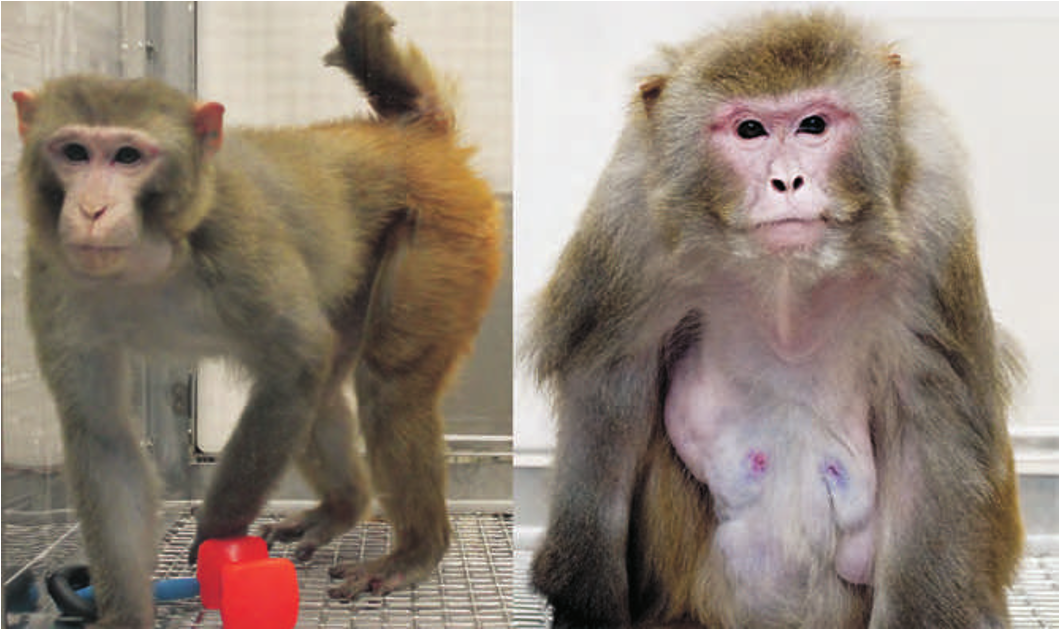
Nonhuman primates are the genetically closest relatives to humans and, as such, are the ultimate model for investigating the biological basis of human aging. Several species of nonhuman primates, such as lemurs, marmosets , monkeys, and great apes, have been used to study the biology of aging, but the majority of well-controlled laboratory studies involve the rhesus macaque (Macaca mulatta) monkey. Aging rhesus macaques display many of the time-dependent physiological declines that are also observed in humans and not often observed in other species (Figure 1.11) . These include visual and auditory deficits, motor function decline, loss in bone mineral content, a true menopause in females and decreasing testosterone levels in males, muscle mass decreases, and a general decline in metabolic function.
Figure 1.11 Rhesus macaque monkeys. Nonhuman primates such as rhesus macaque monkeys are invaluable models for human aging because of their genetic similarity to Homo sapiens. As shown here, rhesus macaque monkeys show visible signs of aging: (A) an 18-month-old monkey; (B) a 25-year-old. They are also susceptible to many age-related diseases and dysfunctions common in humans. (A, courtesy of J. Lenon; B, courtesy of J. Miller.)
Rhesus macaques are also susceptible to many time-dependent human diseases, such as type 2 diabetes, cardiovascular disease, and pseudo forms of both Alzheimer‘s and Parkinson‘s disease. The etiology of type 2 diabetes and cardiovascular disease seems to be identical in rhesus macaques and humans.
The similarity between nonhuman primates and humans in time-dependent functional loss and disease provides scientists with highly controlled populations in which to perform repeated noninvasive or low-risk invasive procedures, testing drugs and other physical therapies. For example, many of the osteoporosis and anti-bone-loss prescription drugs currently available to humans were tested on rhesus macaques.
However, the same physiological similarities that make these species attractive as models for aging also present a major limitation. The genetic similarity to humans has led to questions about the ethics of conducting invasive experiments on such close relatives. Regulatory agencies charged with insuring the humane treatment of research animals have responded by limiting the type of research that can be conducted in nonhuman primates. In general, investigative procedures are limited to those that are also permitted in humans, although the safety standards may be lower (for example, X-ray exposure limits are higher for monkeys than humans).
Another major limitation of using nonhuman primates as models for human aging is cost. Animals must be maintained in highly controlled conditions throughout their life span. The average life span of a rhesus macaque housed in a certified animal facility is approximately 35 years, with a maximum life span approaching 45 years. The average cost for maintaining a rhesus monkey varies with the research institution, but generally ranges from $9 to $10 per day. Thus, maintaining a single rhesus macaque for 35 years would cost approximately $121,000. It is unlikely that an individual investigator could receive sufficient funding to conduct an investigation with the numbers of rhesus monkeys needed to complete a well-controlled study in aging. The NIA, for example, currently supports research in rhesus monkeys at only two locations in the country.
Human progerias can be used to model normal human aging
Werner syndrome and Hutchinson-Gilford progeria syndrome are diseases that many believe are associated with premature aging (Figure 1.12) . Progerias are rare genetic conditions marked by slowed physical growth and characteristic signs of rapid aging. Hutchinson-Gilford progeria syndrome affects people at birth and during young childhood, whereas Werner syndrome normally begins to express itself in the second or third decade of life. While both syndromes place the individual at greater risk for developing age-related diseases, patients who have Werner syndrome tend to die of cancer and atherosclerosis, whereas those with Hutchinson-Gilford progeria syndrome are more prone to cardiovascular and neurological disorders.
Figure 1.12 Woman who has Werner syndrome. The woman is shown (A) at 13 years of age and (B) at 56 years of age. (A, from F.M. Hisama, V.A. Bohr, and J. Oshima, Sci. Aging Knowl. Environ. 10:18, 2006. With permission from the American Association for the Advancement of Science. B, courtesy of J. Oshima)
Because people with Werner syndrome tend to have longer life spans than those with Hutchinson-Gilford (45–50 years vs. 12–15 years), Werner syndrome is considered a better model for aging. There are four features common to all individuals affected with Werner syndrome: short stature, early graying and loss of hair, cataracts in both eyes, and scleroderma-like skin changes. Many affected individuals also have flat feet, changes to their voice, and hypogonadism. People with Werner syndrome have a high risk for developing type 2 diabetes, atherosclerosis, coronary heart disease, hypertension, and osteoporosis. These changes in appearance and increased risk for age-related diseases are clearly similar to those that happen during normal aging.
Werner syndrome is caused by mutations in the WRN gene. This gene is responsible for production of the WRN 蛋白质, which plays a role in the maintenance and repair of DNA. The 蛋白质 may also assist in DNA replication. The lack of or decreased function of the WRN 蛋白质 mimics the outcome predicted in some theories of aging that are discussed in Chapter 4.