4.3 复制导致的衰老
细胞衰老理论预示了细胞功能改变的调控或“驱动”了生物的衰老。因为生物的衰老表现在生殖之后的功能逐渐衰退,功能衰退和细胞失去分裂能力被视为细胞衰老的特性,生殖之后的时期成为复制衰老(replicative senescence)。本部分我们探究细胞是否有一个有限的寿命,如果有,那么它是如何影响整个生物体的衰老或寿命的。
4.3.1 一个错误导致细胞衰老的发现延迟了50年
在1912年,Alexis Carrel去了一小块鸡的心脏,把它放在一个容器里,容器下面是鸡血浆和液化的鸡胚胎组织的混合物。血浆中的蛋白质包含了细胞生长所需的物质。液化的胚胎组织提供了生长所需的营养,尽管我们对这个液体中的化合物并不是完全了解。两天之后,新细胞出现在这片鸡心脏之上。在Montrose Burrows的帮助下,Carrel成为第一个在动物体外成功培养细胞的人。
过了几天,当液化的鸡胚胎组织耗尽时,培养基细胞的生长变慢并停止,一些细胞张到外面去了。Carrel去掉一些细胞,把它们放在一个新容器中,并且添加新鲜的鸡胚胎组织和血清。像之前那样,新的培养基中的细胞继续分裂,直到接触到培养皿的边缘。这些细胞从1912年开始培养,一直持续到1946年Carrel挂掉,这个实验导致了人们认为细胞是永生的。但是科学家们发现Carrel液化鸡胚胎组织的方法并没有将血清中的活细胞全部去掉。也就是说,培养的细胞不是永生的的;它只是在每次更换血清时加入了新的细胞。尽管如此,体外细胞永生的教条被广泛接受,并且在50年内很少受到挑战。
. . . . . . . Indeed, so strong was the belief that cells grown in culture were immortal that evidence suggesting otherwise was viewed as being the result of technical error and universally attributed to a human mistake. Thus, it was understandable why, in 1961, Leonard Hayflick and Paul Moorhead, who worked at the Wistar Institute in Philadelphia, were cautious about reporting their findings that in vitro cultures of human embryonic fibroblasts were dying after several population doublings.
4.3.2 Hayflick和Moorhead的发现从而创建了细胞衰老学
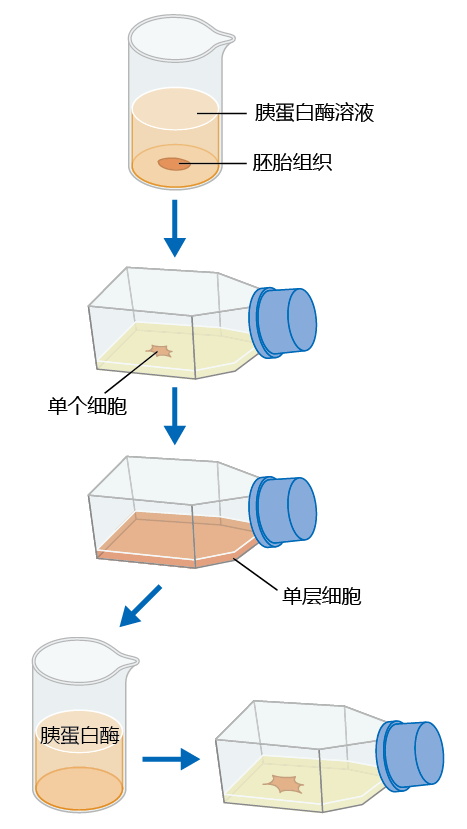
Hayflick and Moorhead were interested in the underlying biochemical mechanism that transformed normal cells into malignant, cancerous tumors. They chose to work with human embryonic fibroblasts (which was legal in 1961) because, as they reasoned, these cells would not have had significant 环境al exposure that might confound the intrinsic biological mechanism(s) responsible for the development of cancer. They no longer grew cells directly on chopped-up pieces of tissue, as did Carrel. Instead, cells from embryonic tissue were separated from each other by the connective tissue–digesting enzyme trypsin and placed on a growth medium in the culture flask (Figure 4.8) . The cells were then suspended in a medium containing the necessary nutrition and cellular growth factors.
Figure 4.8 Method used to determine the life span of cells in culture. Fibroblasts are separated into individual cells by trypsin digestion. The cells are then placed into a culture flask containing cell-free fetal bovine serum. When the cells completely cover the medium in the flask and there is no more room to grow (confluence), they are again digested with trypsin, and a few of the detached cells are re-plated as before. Each passage of cells from trypsin digestion to confluence is referred to as one population doubling.
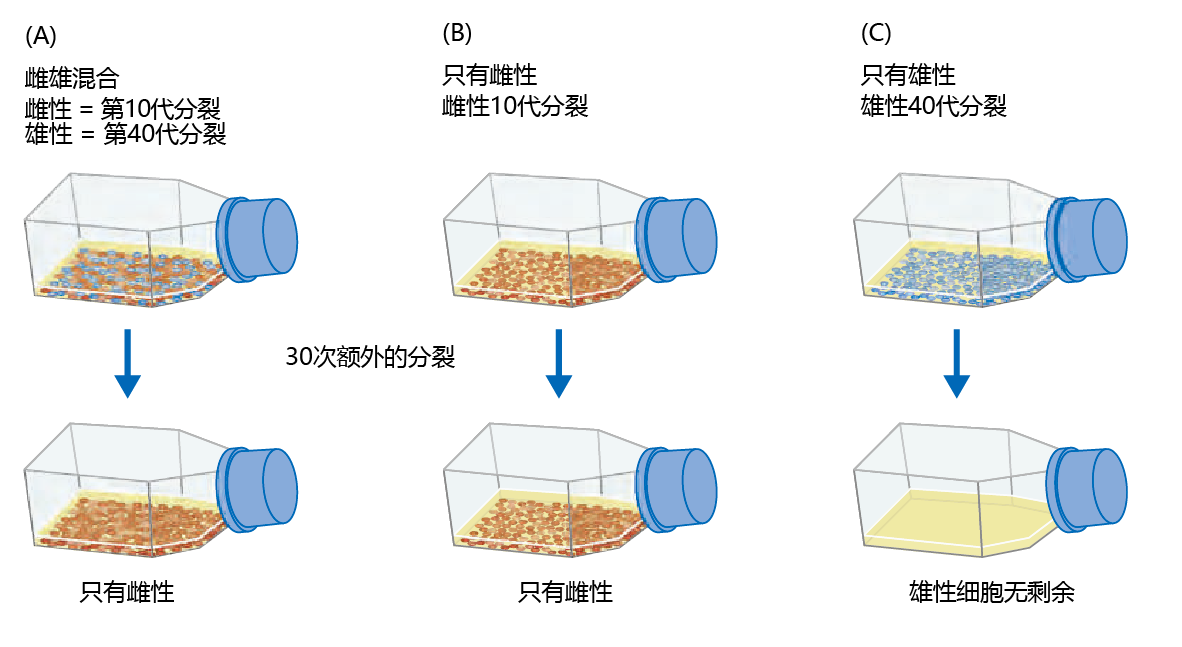
After a few months of working on the experiment, and after several population doublings, Hayflick and Moorhead found that some of the cells were no longer dividing. Meticulous observation showed that subcultivations would cease to divide after 40–60 population doublings. In subsequent years, the finite number of cell population doublings would be known as the Hayflick limit. However, the dogma that cells were immortal was so ingrained in the thinking of biologists that the scientific community did not accept this first experimental evidence of finite cell population doubling. Hayflick and Moorhead received criticism from experts in the field, suggesting that their cell cultures might have chromosomal damage causing the death of the cell population and that this damage might be greater in one sex than the other. In answer to this criticism Hayflick and Moorhead performed one more experiment, in which chromosomes from both sexes were evaluated for structural integrity and whether gender had an effect on survival. In Hayflick's own words, here is what they did (Figure 4.9) :
Figure 4.9 Hayflick and Moorhead‘s experiment to determine whether cells grown in culture have a finite life span. Hayflick and Moorhead tested three different cultures. (A) A mixture of equal numbers of human male cells (blue) in their 40th population doubling and human female cells (red) in their 10th population doubling. After an additional 30 population doublings, only the female cells continued to divide. (B) Female cells started at the 10th population doubling and kept for 30 additional population doublings. Female cells were still dividing after 40 population doublings. (C) Male cells started at the 40th population doubling. No viable male cells were left after 30 additional population doublings.
. . . we mixed equal numbers of human female cells [determined by sex chromosome analysis] at the tenth subcultivation level (young cells) with human male cells at the fortieth subcultivation level (old cells). We also continued to subcultivate the unmixed cell cultures of each sex. . . . After about thirty more subcultivations we looked at all three cultures. In the mixed culture we found only female cells present. The male cells in the mixture had died several weeks before, when they had reached their maximum of fifty subcultivations. The pure male cell culture had died several weeks before we decided to look at the mixture. The pure female cell culture, like the female cells in the mixture, were still luxuriating. (Hayflick & Moorhead 1961)
Little doubt was left that cells grown in vitro did indeed have a finite life span. The prediction of an 进化ary theory of senescence with a finite life span for cells was, at least for cells in culture, accurate. So important were these results that a new field of study was born—cytogerontology, the study of cell senescence (now known as replicative senescence).
4.3.3 Cells in culture have three phases of growth
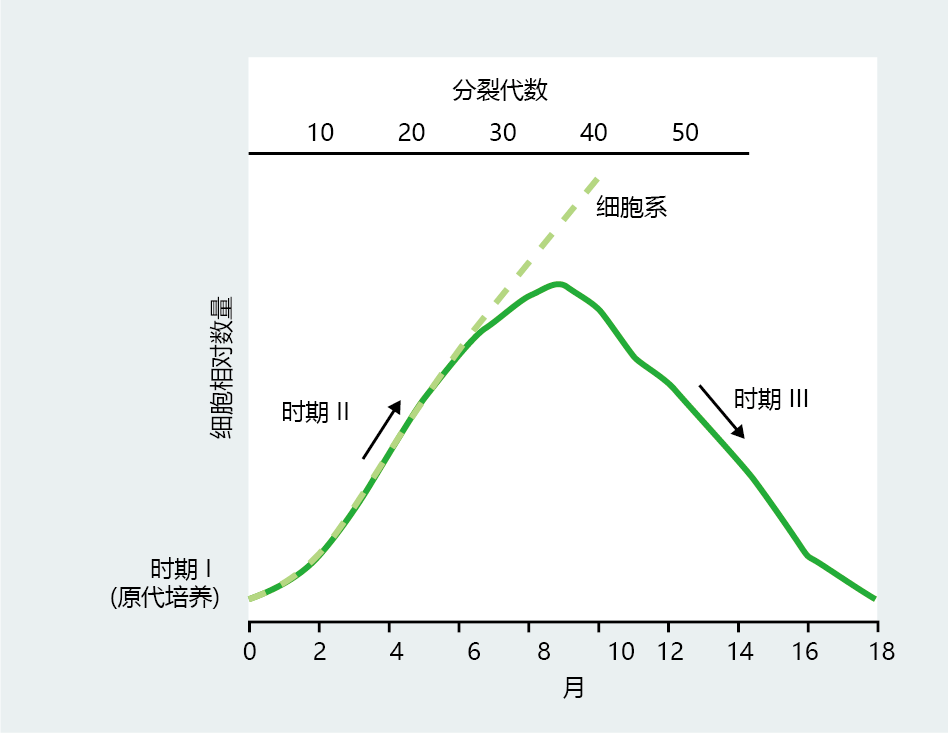
Beyond proving that cells were mortal, Hayflick and Moorhead's experiments established, for the first time, the life-span characteristics of cells in culture. Cell cultures derived from a small population of homogeneous embryonic cells grow in three distinct phases, as shown in Figure 4.10 . Phase I is characterized by a rather slow growth period occurring immediately after the cells are removed from the donor and plated on the first growth medium. This slow growth may last for 2–3 months, during which the first 10–12 population doublings may occur. Phase I most likely reflects a period of adaptation of the cells to the new in vitro 环境. The slow growth of phase I gives way to the rapid and constant proliferation rate of phase II, which may last for an additional 8–9 months of cell division (30–40 population doublings). During phase II, mutations can be introduced that change the culture from one with a finite life span to one with an infinite life span. Finally, the cell culture enters phase III, a period of declining cell proliferation rate that ends in the loss of cell division. Hayflick and Moorhead originally proposed that phase III ends with the culture's loss of proliferation at about 12 months. Subsequent studies found that a subpopulation of cells will cease replication but remain physiologically viable for extended periods of time.
Figure 4.10 Life history of mitotic cells in culture, as originally described by Hayflick and Moorhead. Phase I begins with the primary culture and is characterized by a slow rate of proliferation and population doubling. Cells in phase II grow rapidly to a peak at about 75% of their maximal population doubling. Cells in phase II can immortalize spontaneously or through an intervention and become a cell line. An increase in population doubling time and the death of the culture occur in phase III. (From L. Hayflick and P.S. Moorhead, Exp. Cell Res. 25:585–621, 1961. With permission from Elsevier.)
The criterion for the end of the replicative life span of a cell population in culture has been widely accepted as the failure of the population to double within four weeks. This definition does not mean that all cells in the population have lost the capacity to divide, just that the majority have. Cells, like whole organisms, age at non-uniform rates. Indeed, in old, non-doubling populations, many individual cells retain the capacity for division. (The opposite is also true: young populations that have doubled only a few times have some cells that are no longer capable of division.) If clones are removed from the flask in which the population has not doubled within four weeks, new populations can be cultivated that have the replication characteristics of phase II. That is, the cell population defined as having reached the end of its proliferative life span may contain individual cells that are still capable of division. In fact, some of the clones will start populations that have the same proliferative life span as the population from which they were derived. Much like the life-span characteristics of a human population, cell populations have significant variation in individual rates of aging and individual life span.
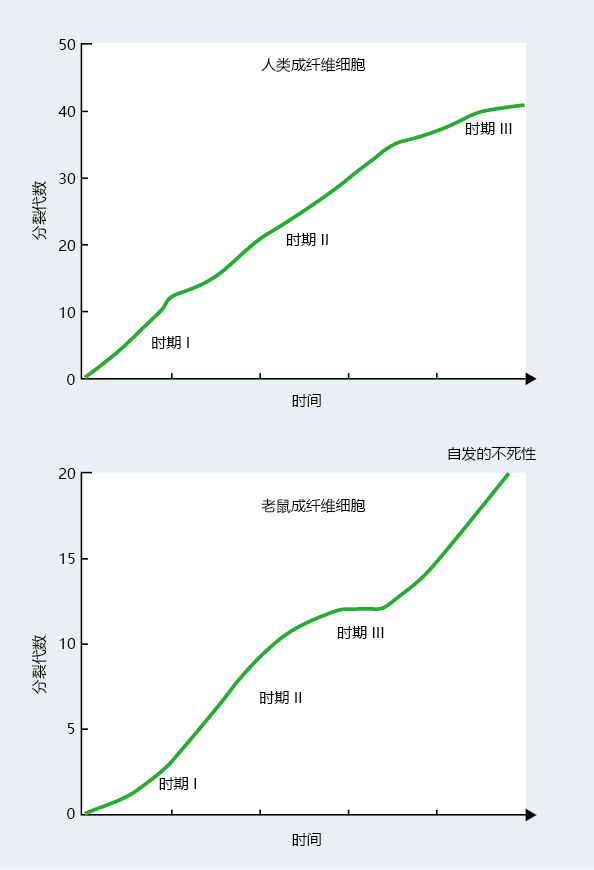
Interspecies comparisons suggest that population doubling rates vary with the species (TABLE 4.2) . Moreover, population doubling rates do not seem to be strongly correlated with length of life. The common laboratory mouse lives an average of 3–4 years, with fibroblasts doubling about 15 times. Human beings have a maximum life span of 115–120 years and have fetal fibroblasts that double 45–60 times (Figure 4.11) . However, the chicken, with a maximum life span of 6–10 years, has a population doubling time closer to that of the long-lived human than the short-lived mouse.
图4.11 Fetal fibroblast doubling times in hypothetical populations of human and mouse cells. Human fibroblast cultures have a maximum of 45–50 doublings. Conversely, the pace of cell division in mouse fibroblasts slows after 15–20 doublings, and then the cells often spontaneously immortalize.
TABLE 4.2 FETAL FIBROBLASTS' POPULATION DOUBLING RATES AND MAXIMUM LIFE SPAN FOR SEVERAL SPECIES
Not all cell types have a finite population doubling time. Fibroblasts isolated from some mouse tissues display infinite population doubling while in phase II growth, a process known as spontaneous immortalization. Primate and avian cells rarely immortalize spontaneously in culture, but they can become immortal through manipulation of their genomes. For example, transfection (the introduction of DNA from the cells of one species or virus into a cell of a different species) of human or other primate fibroblasts by the Simian virus 40 (SV40) T-antigen gene leads to immortalization of the population. As discussed in BOX 4.1, cancer cells also undergo an infinite number of population doublings. Cells that are immortal are referred to as cell lines and have found wide use in biology because of their ease of replication and uniform genetic makeup.
| BOX 4.1 THE CELLS OF HENRIETTA LACKS: HERS OR OURS? |
|
Techniques employed by modern molecular cell biologists have provided immortal cell lines for just about every conceivable research question. Cell lines constructed by genetic engineering or started from malignant tumors can be obtained commercially or through cell-line repositories maintained by the national government. There was a time, however, when such cell lines did not exist. That all changed on a winter's day in 1951. Henrietta Lacks, 34, mother of five, arrived at a Baltimore hospital on February 9, 1951, to begin radiation treatment for a malignant cervical tumor. Before the physician covered the tumor with radium, George Gey, a resident at the hospital, requested a small sample of the tumor. Gey was interested in how cancer cells grow, and he had not been able to culture a long-term colony of cells from other tumor types. Henrietta Lacks‘s cervical tumor cells were different. Within hours after the cells were placed on a growth medium, they began to grow. Gey soon found that he could culture these cells indefinitely, and he began to offer subcultivations, which he named HeLa for Henrietta Lacks, to other scientists. Indeed, it was HeLa cells that Jonas Salk used to test his polio vaccine prior to human testing. Henrietta Lacks died on October 4, 1951, but HeLa cells remain the most widely used cell line in the world and continue to provide scientists with a reliable tool for specific research questions. Oddly, however, it was not until 1975 that David Lacks, Henrietta's husband, and the rest of the Lacks family found out about HeLa cells. Quite possibly, the only reason the family found out was that a need arose for the Lacks family's DNA. HeLa cells were so widely used that they began to contaminate other cell lines. Researchers needed DNA from Henrietta Lacks's close relatives to develop an assay that could distinguish HeLa cells from other cell types. There is no doubt that many people and companies have profited from the use and/or sale of HeLa cells. The Lacks family, however, has not realized a penny from Henrietta‘s cells, nor is it likely they ever will. The US Supreme Court ruled that cells removed from an individual are no longer the property of that individual. Rather, the cells and/or tissue belong to the person responsible for the removal of the cells. Particulars as to who will profit from the sale of the tissue must be specified at the time of informed consent. Because there was no informed consent when Henrietta‘s cells were removed, the Lacks family is not entitled to compensation. |
4.3.4 Senescent cells have several common features
Replicative cessation is the major characteristic of late-passage cells— cells that are part of a population approaching its Hayflick limit—but several other features are also commonly observed in senescent cell populations, including morphological alterations, cell function alterations, arrest in cell division, and immunity-associated functions (TABLE 4.3) . Senescent cells typically display cellular enlargement, including an increase in size of the nucleus and multinucleated cells. Moreover, the distance between cells becomes greater, due to an increase in the cellular secretion of extracellular matrix proteases and collagenase. The decrease in viable extracellular matrix 蛋白质 may also cause a slowing of replication, as the cell will not have sufficient material to anchor itself.
TABLE 4.3 PHENOTYPE OF A SENESCENT CELL POPULATION
The slowing of replication seems to be due to a lengthening of the 细胞周期 that most likely reflects the activation of molecular brakes at the G1 -S phase interface. This inhibition of replication in senescent cell opulations during G 1 is supported by the observation that the intracellular mitogenic signal remains responsive to extracellular mitogens. Such observations have led to the suggestion that cell senescence may be caused by errors in the replication mechanism, although consistent data supporting this theory have yet to be obtained.
Cells in a senescent population undergo a general decrease in function that includes decreases in the rate of DNA, RNA, and 蛋白质 synthesis.
Given that the synthesis rates of these vital cell components regulate, to a large degree, the overall function of the cell, it is not surprising to find a general decrease in physiological function in senescent cell populations. Moreover, senescent cell populations are characterized by an increase in intracellular “junk.” This so-called junk reflects a decrease in the ability of senescent cells to break down and metabolize non-functional 蛋白质. Finally, senescent cells seem to have an increased secretion of 蛋白质, such as inflammatory cytokines, normally found in cells that have been injured.
4.3.5 Replicative senescence can be used to describe 生物学衰老
From the first description of cellular senescence provided by Hayflick and Moorhead, many scientists have questioned the value of an in vitro model as an appropriate descriptor of aging in whole organisms. While there can be little doubt that an organism‘s aging must reflect cellular dysfunction, the fact remains that most cells in adult eukaryotic organisms are post-mitotic. Thus, it is not clear how a model of
aging based on the cessation of cell division adds valuable information about a process that occurs mainly in the absence of cell division. Although supporters of replicative senescence often point to the fact that population doubling rates correlate with a species‘ life span, these correlations are weak at best and do not occur in all species. In addition, mitotic somatic cells taken from old individuals can often show robust population doubling rates.
Most biogerontologists would agree that cellular replicative senescence, per se, does not directly cause aging in organisms that are primarily post-mitotic. Evaluating the relationship between cellular replicative senescence and organismal aging is not, however, the only value of using cell cultures as a model of aging. Rather, cell cultures can provide researchers with a highly controllable and predictable 环境 in which to describe a variety of aging events at the cellular level that are not directly related to the population‘s replicative history. We are just beginning to see results from investigations using the cellular replicative senescence model to evaluate alterations in membrane function, mitochondrial damage, and other phenomena related to rate of aging rather than longevity. One exciting area of research where replicative senescence has the potential for offering significant value is the interface between aging and disease. These investigations, only recently begun, are asking the important question, “What are the molecular mechanisms that underlie a cell's transition between normal function and physiological states that increase the risk of disease?”