4.4 引起细胞衰老的原因:被破坏的生物分子的积累
The mechanisms underlying replicative senescence are not yet known and remain at the theoretical level. Theories providing an explanation for the process of replicative senescence fall into two categories, depending on the process being described and the 进化ary evidence underlying that mechanism. If mechanisms leading to loss of function with age are the main concern, replicative senescence will be described by random or stochastic events. If cell longevity is the primary concern, there should be genetic or programmed mechanisms leading to the death of the cell. In later sections, we explore random-event mechanisms (“Oxidative Stress and Cellular Aging”) and a programmed theory of replicative senescence (“Telomere Shortening and Replicative Senescence”).
Most biogerontologists agree on one point: cellular aging is the result of an intracellular accumulation of damaged 蛋白质. Therefore, in this section, we explore how and why the accumulation of damage to biomolecules occurs and why this damage may lead to altered rates of aging.
4.4.1 Biomolecules are subject to the laws of thermodynamics
Like all matter in the universe, biomolecules are subject to the laws of thermodynamics, physical laws governing the relationship between work, energy, and heat. The first law of thermodynamics applies to the conversion of energy from one form to another. When converting energy from one form to another, the total energy of the system before and after the conversion remains the same. That is, energy is neither created nor destroyed. In the conversion of energy from one form to another, we run up against the second law of thermodynamics: the transfer of energy from one form to another is not 100% efficient, and some of the energy becomes unusable. Unless the system receives an input of new usable energy to replace the amount of unusable energy lost as heat, the system will become randomly rearranged—that is, will move in the direction of disorder (entropy). It is this movement toward increasing entropy that causes the accumulation of damaged 蛋白质 that leads to aging. The total energy of a biological process is called enthalpy (H) and equals usable energy (free energy, G) plus unusable energy (entropy, S). This relationship is described in Equation 4.1.
Total energy of a system, H = G+ TS
Where
H = enthalpy, the total energy in a system
G = usable energy, or free energy
S = unusable energy, or entropy
T = temperature of the system
Because the energy resides in the bonds of molecules, H, G, and S cannot be measured directly in a living biological system. We can, however, measure the change, represented by the Greek letter delta (Δ), in each quantity, as long as we know the temperature at which the reaction occurs. We start by determining whether a reaction releases or consumes usable energy. That is, ΔG = G products − G reactants . If the reaction increases usable energy, the sign of ΔG is positive; if the reaction decreases usable energy, the sign of ΔG is negative. Since the first law tells us that the total energy of a reaction does not change, any change in G must be accompanied by an equal and opposite change in H and S, but H cannot be measured. Therefore, we can measure ΔG and ΔS and solve for ΔH (Equation 4.2) .
Change in usable energy of a chemical reaction,
ΔG = ΔH − TΔS
Where
ΔG = change in free energy of a reaction (G products − G reactants )
ΔH = total amount of energy added to or released by the system
ΔS = change in entropy
T = temperature of the system
4.4.2 Life requires the constant maintenance of order and free energy
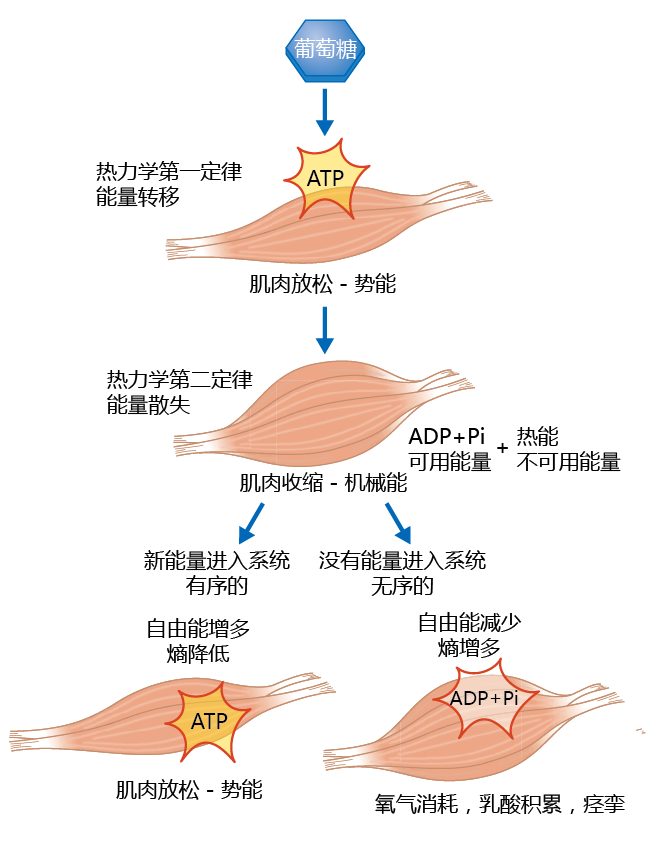
Biological processes use molecules with significant usable energy (highly ordered) in reactions that result in an organism‘s productivity, growth, and repair. In these reactions, and because of the second law, unusable energy is released, which lowers the amount of usable energy needed to carry out additional biochemical reactions that sustain life. As usable energy decreases, entropy and the disorder of the system (or unusable energy) must increase, according to the first law. To restore the order, and thus maintain life, organisms must constantly supply new usable energy to replace that lost to entropy. Let‘s use the contraction of a muscle to illustrate this principle of thermodynamics in biological processes. Muscle cells (muscle fibers) convert the chemical energy of adenosine triphosphate (ATP) into mechanical energy called contractions (图4.12) . The conversion of ATP to ADP (adenosine diphosphate)+ P i (inorganic phosphate) releases usable energy that drives the contraction. Because of the second law of thermodynamics, unusable energy in the form of heat is also released. In fact, 80% of the energy derived from breaking the phosphate bond is released as heat; entropy and disorder increase and usable energy decreases with every contraction of a muscle fiber. If the system provides a mechanism that replaces the usable energy lost to entropy, then the muscle can relax in preparation for the next contraction. That is, the order lost to entropy is restored, as seen in Figure 4.12, with conversion of ADP to ATP (that is, more bonds, more usable energy, more order). Conversely, if that energy lost to entropy cannot be restored, the muscle fiber remains in the contracted state, resulting in a lack of oxygen, and cells will begin to die. If additional free energy never comes—that is, if we are dead—the laws of thermodynamics work to completion and the muscle fiber achieves energy equilibrium.
Figure 4.12 The first and second laws of thermodynamics applied to muscle cells. Muscle cells convert glucose energy into ATP energy in preparation for contraction, as dictated by the first law of thermodynamics. The conversion of ATP to ADP+ P i releases usable energy that drives contraction. Because of the second law of thermodynamics, heat is also released. If more glucose enters the system, replacing energy lost to contraction, the muscle relaxes in preparation for the next contraction. If no glucose is available for conversion to ATP, the muscle remains in the contracted state.
4.4.3 The 机制 underlying 衰老 is the loss of molecular fidelity
Recall that the atomic structure of a molecule determines its functions. We know this principle as the structure-function relationship. If even one amino acid in a 蛋白质 does not appear in the proper sequence, then the biological activity of that 蛋白质 can be reduced. Maintaining the proper structure of a molecule, known as molecular fidelity, is critical for survival of the organism and requires a constant input of usable energy. In other words, organisms are constantly battling the increase in entropy, and successful organisms are those that win the battle by maintaining molecular fidelity.
The ability to restore the free energy lost to entropy and maintain order requires an enormous investment of resources by the organism in order to survive to reproductive age. At the very basic level, successfully surviving to reproductive age simply reflects genes that have been selected to maintain the molecular fidelity of 蛋白质. However, as you learned in Chapter 3, there would be no reproductive advantage to maintaining this high level of investment in resources after reproductive age has been achieved. The laws of the universe would eventually rule,
and the slow march toward energy equilibrium would lead to a loss in molecular fidelity, disorder of the cell, and a decrease in cell function due to accumulation of damaged 蛋白质. Cellular aging reflects the accumulation of damaged 蛋白质 caused by the basic laws of the universe—the disorder of increasing entropy.
4.4.4 Aging reflects the intracellular accumulation of damaged biomolecules
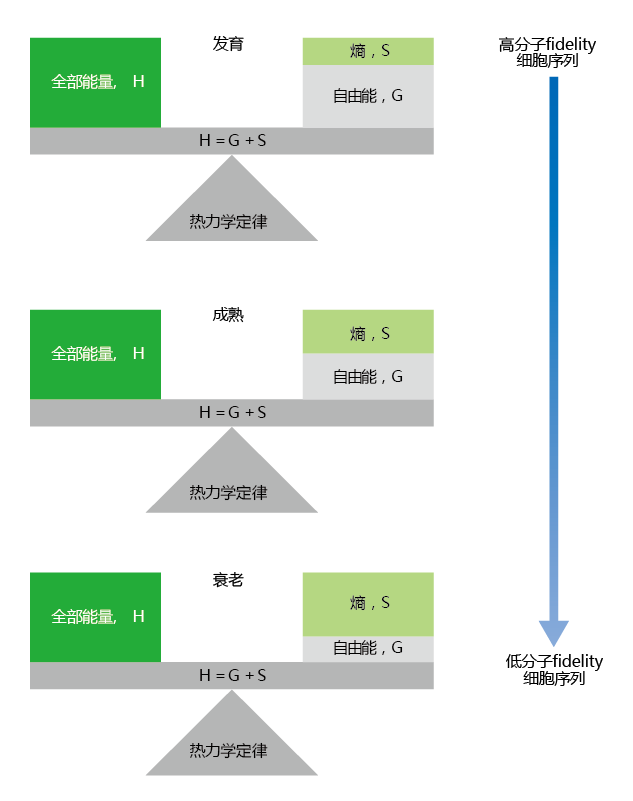
There is now overwhelming evidence to indicate that the passage of time results in changes to molecular structure. While the mechanisms underlying these changes remain unclear, we can say with confidence that the cellular accumulation of damaged biomolecules “speeds up”after sexual maturation. Since structure determines function, the older cell becomes less efficient in carrying out normal operations. We can think of somatic cellular aging in terms of a balancing act between the maintenance of molecular fidelity (order) and entropy (disorder) (Figure 4.13) . Due to the force of natural selection, genes have been selected that maintain molecular fidelity during development, a period in which highly functional cells are critical. With the passage of time, the extrinsic rate of aging, in accordance with the disposable soma theory (see Chapter 3), causes damage to biomolecules. Because the 蛋白质 that help to maintain molecular fidelity are also subject to
the second law, the ability to repair or replace damaged biomolecules declines, resulting in the intracellular accumulation of damaged biomolecules over time. When entropy begins to outpace the maintenance of molecular fidelity, the cell can no longer maintain normal function, and cell death occurs.
Figure 4.13 Molecular fidelity and energy balance during the life span. Molecular fidelity and cellular order are maintained during the run-up to reproductive age (development). Replacement of usable energy outpaces the amount of energy lost to entropy. As we age beyond the start of reproduction (maturity), increasing entropy leads to a loss in molecular fidelity and the accumulation of damaged 蛋白质—cellular disorder. Senescence is characterized by low molecular fidelity and increased cellular disorder, as entropy increases faster than the introduction of new usable energy.
We have been purposely vague about the precise type of damage that may cause the molecular infidelity leading to cellular aging. The reason for this is that the exact type of damage occurring to the cell is largely unimportant to the process of aging. Both the type and amount of damage vary considerably among cell types, and no single type of damage process can be considered the primary cause of cellular aging. For example, in this chapter we use damage caused by oxygen-centered free radicals to describe how random events in normal metabolism lead to the accumulation of damage and the appearance of aging. Other chapters describe how the addition of glucose to 蛋白质, known as glycosylation, changes the 蛋白质‘s structure and leads to accumulation of damage. In Chapter 9, you will learn that the misfolding of 蛋白质 can result in insoluble aggregates that may be the precursors to neurological disease. It would be difficult to demonstrate that age-related damage caused by oxygen-centered free radicals is more important to the aging process than are glycosylated 蛋白质 or insoluble 蛋白质 aggregates.
The important message to take away from this discussion of the cause of the accumulation of damage is not what type of damage accumulates but that cellular damage occurs in accordance with the basic laws of the universe. As such, we have no more control over the deteriorating effects of aging within the human body than we do with any other object in the physical world. We may be able to stop one type of damage, only to find that another type has started. This will continue until the cell succumbs to the second law. Death of the organism occurs when enough cells of a particular organ or organs have stopped functioning as a result of molecular infidelity. You will learn in Chapter 10 that there are ways in which we can slow the rate of aging and live longer, healthier lives, but aging will eventually prevail and the universe will claim us.