5.5 GENETIC 调控OF 寿命 IN C. ELEGANS
The nematode C. elegans, just 1 mm long, lives in the soil in several climate zones; it has been used as a model organism for the genetics of longevity for more than 30 years. This roundworm feeds primarily on bacteria, but it can be grown in the laboratory on several different growth media.
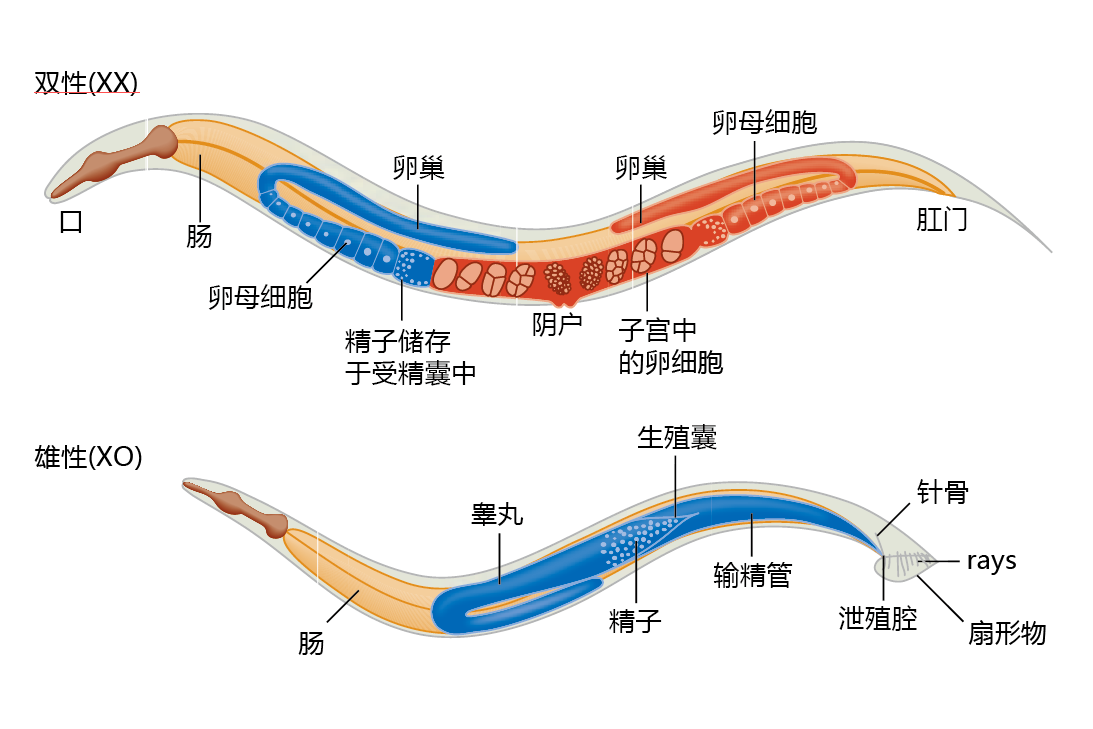
There are two sexual forms of C. elegans, hermaphrodite and male. Hermaphrodites produce both sperm and eggs and reproduce by self-fertilization (Figure 5.24) , whereas males produce only sperm. Males arise spontaneously at very low frequency (1 in 500) and can fertilize hermaphrodites; hermaphrodites cannot fertilize other hermaphrodites.
Figure 5.24 The two sexual forms of C. elegans: hermaphrodite and male.
All 959 cells of the adult hermaphrodite are post-mitotic; the adult male consists of 1031 post-mitotic cells. Being completely post-mitotic and having a significant post-reproductive life span makes C. elegans an exceptional model for studying the genetic regulation of longevity in multicellular organisms. Another important advantage in using C. elegans is that the lineage and function of every cell are known. Interestingly, approximately one-third of the cells are nerve cells, which makes C. elegans especially useful for describing cell regulatory and signaling pathways.
The genome of C. elegans has approximately 100 million bp, eight times the size of the yeast genome and about three-quarters the size of the Drosophila genome. C. elegans has six chromosomes, five pairs of autosomes and one sex chromosome. Hermaphrodites have two sex chromosomes (designated XX). Males have one X chromosome (designated XO). Males cannot produce progeny on their own. However, they can cross-fertilize hermaphrodites. The sequence of the genome was completed in 1998 and has approximately 20,000 蛋白质-coding genes. In this section, we examine the dauer formation gene (daf-2), along with other genes associated with daf-2, and the clock genes.
5.5.1 Regulation of dauer formation extends life span
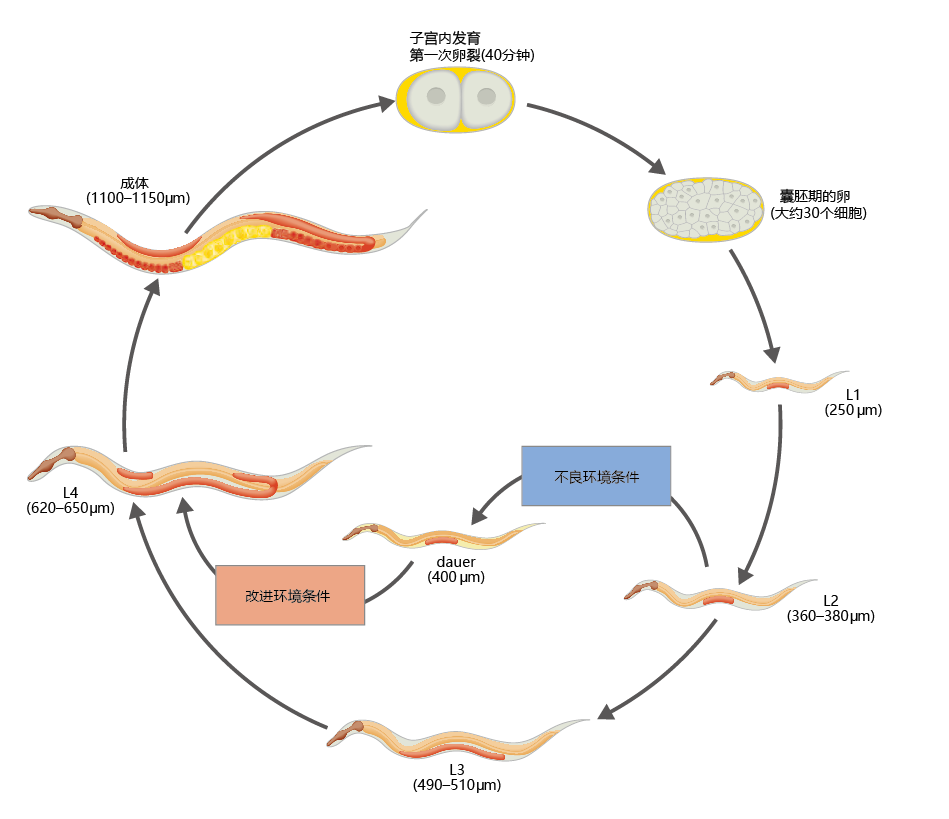
Development from egg to adult in C. elegans consists of four larval stages and takes about 3–4 days to complete. Adults are reproductively active for the first 4 days of adult life and may live an additional 10–15 days after this 4-day reproductive period. When C. elegans finds itself within an 环境 that will support the survival of offspring, the worm progresses through the four larval stages to adulthood in the normal 3- to 4-day period. However, if 环境al conditions are less than optimal for reproduction, development is halted at larval stage 3 and a metabolically active but reproductively silent larva called the dauer forms (Figure 5.25)
Figure 5.25 Developmental stages of C. elegans. Eggs are fertilized in the adult hermaphrodite and laid a few hours later—at about the 30- to 40-cell stage. After the eggs hatch, the worms pass through four larval stages (L1, L2, L3, and L4), each of which ends in a molt. On reaching adulthood, each C. elegans produces about 300 progeny. Its life span is about 2 weeks. If 环境al conditions are less than optimal in stage 1, growth is arrested at stage 3 and a dauer forms. C. elegans can remain in this arrested state of development for several months. When 环境al conditions improve, the dauer resumes development into the adult worm.
The sexually immature dauer is smaller than its stage-3 counterpart in the normal growth cycle, and it can survive for several months without food and in temperatures and soil conditions that are less than optimal for reproduction. It accomplishes this feat by reducing its metabolic rate, limiting 蛋白质 synthesis, and surviving on stored fat. Anatomical changes to the dauer increase its resistance to stress and thereby increase its chance of survival. These changes include (1) increasing the thickness of its cuticle; (2) closing the buccal (mouth) cavity; and, in some cases, (3) increasing the concentration of endogenous antioxidants. So effective are these morphological alterations that the C. elegans dauer can survive several hours of insults from detergents, radiation, and a host of other noxious agents. Nonetheless, the dauer can rapidly resume development when 环境al conditions improve. Genetic and biochemical mechanisms allow the dauer to re-enter the larval stages within one hour of placement of food in its 环境; molting to larval stage 4 occurs within just eight hours after feeding.
5.5.2 Genetic pathways regulate dauer formation
Several 蛋白质 have been identified as important in formation of a C. elegans dauer, as shown in TABLE 5.2 . The genetic pathways involved in formation of the dauer and its ability to survive under extreme stress have provided some important insights into at least one mechanism that can extend life span.
TABLE 5.2 BRIEF DESCRIPTION OF 蛋白质 IDENTIFIED AS IMPORTANT IN FORMATION OF A DAUER AND EXTENSION OF LIFE SPAN IN C. ELEGANS
Genetic breeding of C. elegans mutants has identified two genes in the same signaling pathway—age-1 (so named for its aging phenotype) and the dauer formation gene, daf-2—that are required for normal growth and reproduction. Without these genes, C. elegans forms dauers. Cloning of the age-1 gene shows its 蛋白质 product (AGE-1) to be a member of the highly conserved phosphatidylinositol-3-kinase (PIK-3) family. These 蛋白质 are important intracellular intermediates between membrane receptors that initiate a signal and the desired intracellular action. Cloning of the daf-2 gene reveals that it encodes a transmembrane receptor 蛋白质 (DAF-2) that has homology with an insulin/insulin-like growth factor (IGF-1) receptor found in other species. A highly conserved gene, daf-2, like its genetic orthologs, is important for normal growth and reproduction.
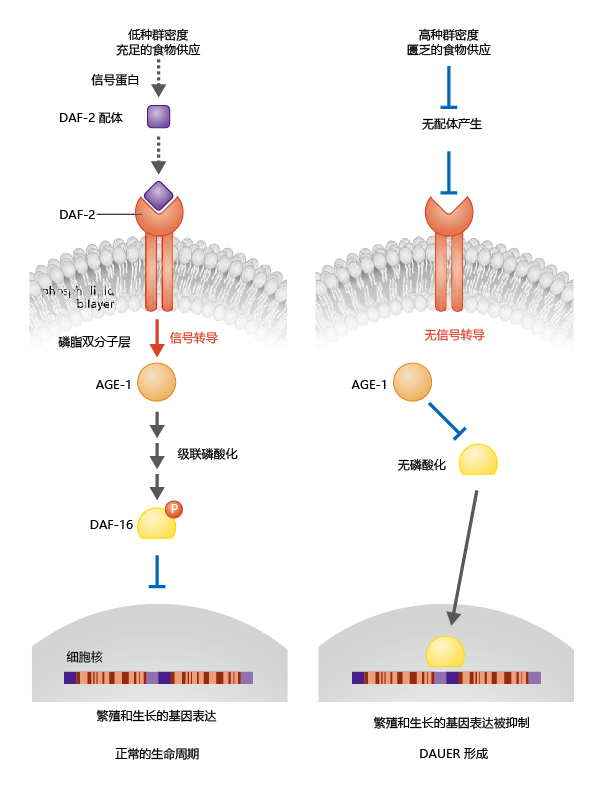
During times of 环境al plenty, signals transferred to the worm result in expression of a DAF-2-binding 蛋白质, for which the precise structure, kinetics, and specific ligand remain largely unknown (Figure 5.26) . Binding of the DAF-2-binding 蛋白质 to the DAF-2 receptor causes the AGE-1 蛋白质 to migrate from the interior of the cytoplasm to the cell membrane. This finding provides an important clue that dauer formation is regulated by the repression of genes tied to an endocrine signal. That is, phosphatidylinositol-3-kinases are activated largely in response to hormones.
Figure 5.26 Regulation of gene expression in adult reproduction and dauer formation in C. elegans. Optimal 环境条件 stimulate the binding of an insulin-like ligand to the DAF-2 receptor (left). The signal propagates through the membrane and attracts intracellular AGE-1, a phosphatidylinositol-3-kinase, to the membrane, where the kinase initiates a phosphorylation cascade. The cascade ends with phosphorylation of DAF-16, a class of 蛋白质 involved in regulating reproduction. The phosphorylation of DAF-16 prevents its entry into the nucleus, and in the absence of DAF-16, genes involved in reproduction and growth can be expressed. Poor 环境al conditions prevent the phosphorylation of DAF-16, which enters the nucleus and represses genes involved in reproduction (right); this results in dauer formation.
The next major advance in our understanding of dauer formation and its relationship to life-span extension in C. elegans came when researchers discovered that phosphorylation of DAF-16 was required for normal growth and reproduction. As shown in Figure 5.26, phosphorylation of DAF-16 prevents its migration from the cytoplasm into the nucleus. As a result, DAF-16 cannot exert its inhibitory effect on genes involved in growth and reproduction; C. elegans progresses normally through the four larval stages into adulthood and exhibits the normal life span of 10–15 days. If, on the other hand, 环境al conditions are inadequate to support reproduction, the DAF-2-binding 蛋白质 is not synthesized and no signal is transduced through the DAF-2 receptor to AGE-1. The DAF-16 蛋白质 is not phosphorylated, and the 蛋白质 enters the nucleus, where it suppresses genes that regulate growth and reproduction. If the repression of genes by DAF-16 occurs during larval stage 3, a dauer forms.
In addition, cloning of the daf-16 gene has shown that the DAF-16 蛋白质 is a member of the forkhead box transcription factor family (FOXO), and that this particular transcription factor represses genes that govern growth and reproduction in C. elegans in response to low-food conditions. FOXO is a highly conserved group of 蛋白质 that are involved in reproduction in many different species, including several mammals.
5.5.3 Weak mutations in the DAF-2 receptor extend life span
Weak mutations in critical genes regulating dauer formation result in an extended adult life span. Weak mutations are alterations in a gene that result in expression being reduced rather than completely eliminated. When weak mutations are induced in daf-2, phosphorylation of DAF-16 is reduced but not prevented. Stage-3 larvae have slightly reduced metabolism compared with the wild type and do not commit to dauer formation. Rather, the reduction in DAF-16 phosphorylation allows C. elegans to progress normally through the four stages of larval development into the adult worm. While the progression to adulthood may be normal, the effects of the mutation are extraordinary: The adult worm with the weak mutation in daf-2 lives 50–300% longer than the wild-type C. elegans.
The C. elegans extended-life-span mutant appears and behaves, for the most part, just like a normal-life-span adult. The adult worm remains fertile, and the hermaphrodite produces only slightly fewer offspring, although some slight modification to the mutation can result in infertility, indicating a close relationship between reproduction and extended life span. Unlike the dauer, the adult worm containing the weak mutation in daf-2 eats normally and responds vigorously to changes in the 环境, including temperature and touch.
5.5.4 Life extension is linked to neuroendocrine control
Cloning of the daf-2 gene and identification of the DAF-2 蛋白质 as an insulin/IGF-1-like receptor indicates that gene regulation leading to extended life span of the adult worm is under neuroendocrine control. Further evidence of neuroendocrine regulation of daf-2 came when the signal transduction pathway was found to involve a PIK-3 (AGE-1), a phosphorylation mechanism often found in hormonal regulation of the cell cycle. Elegant experimentation in which the daf-2 mutation was localized to cells of different lineages—neural and muscle cells—confirmed that the 环境al sensing and signal transduction involved in the daf-2 mutation leading to extended life span were limited to neural tissue. When the mutation was limited to only muscle cells, there was no increase in longevity. However, limiting the mutation to neuroendocrine cells increased longevity. That is, neuroendocrine mechanisms seem to regulate dauer formation and extended life span in C. elegans. The hormonal control of longevity is discussed in detail in the next two sections, for D. melanogaster and M. musculus.
5.5.5 Mitochondrial 蛋白质 may be the link between extended life span and metabolism
A direct link between energy metabolism and life-span extension in C. elegans has been identified with another gene regulatory pathway, found in mitochondria—the clock genes (clk-1, clk-2, clk-3, and gro- 1). Clock genes are a highly pleiotropic set of genes, so named because they regulate the timing and synchronization of several mitochondrial functions. Loss-of-function mutation in clk-1 affects several systems and results in a “slowing down” of many functions, including larval development, egg production, and brood size. The slowdown in function of the clk-1 mutant also results in a slightly smaller worm that lives 15–30% longer than the wild-type C. elegans. Because of its pleiotropic nature, the clk-1 genetic pathway most likely to be associated with the increase in longevity has not yet been identified.
The most commonly accepted theory concerning the clock genes links a slowdown in metabolic rate to extended longevity. The Clk-1 protein has been identified as a demethoxyubiquinone mono-oxygenase (DMO), an enzyme required for the biosynthesis of ubiquinone, also known as coenzyme Q (CoQ). Coenzyme Q is found in high concentration in the inner membrane of mitochondria, where its acts as an electron transfer 蛋白质 between the flavo蛋白质 and cytochrome b. The loss-of-function mutation in clk-1 that results in diminished levels of CoQ slows, but does not stop, the transfer of electrons through the ETS. In turn, energy reserves fall and physiological functions must slow down.
The pathway that regulates the gene expression of DMO from clk-1 has not been described, nor has the mechanism for life-span extension. However, two theories have been advanced that center on the function of CoQ in mitochondria. One theory suggests that a reduction in CoQ level decreases the amount of ETS activity, which, in turn, reduces the production of oxygen-centered free radicals. A decrease in free radicals would reduce cellular damage and possibly extend life span. This explanation, however, would not account for the pleiotropic effect of the clk-1 gene in the slowing of several, diverse systems. The second theory predicts that the regulatory process in the nucleus “senses” the chronic low energy state of the cell—that is, low ATP—resulting in reduced gene expression of 蛋白质 involved in various physiological functions, a kind of energy thermostat. This theory explains the pleiotropic nature of the clk-1 gene, but falls short of explaining the extended life span. As with many genes shown to extend life span in C. elegans, a more precise explanation of why the clock genes extend life span awaits characterization of the pathways that regulate gene expression.